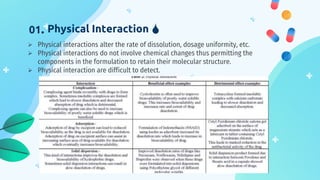
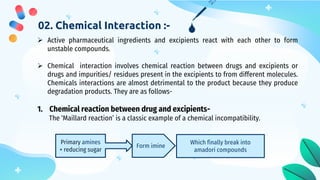
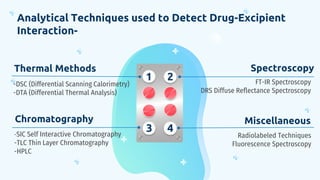
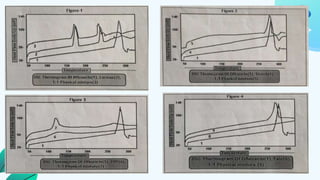
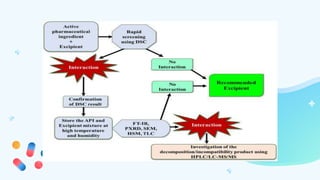
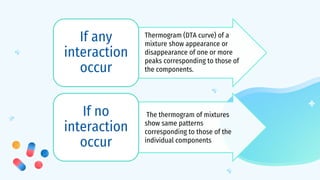
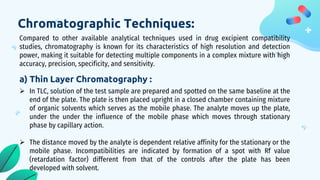
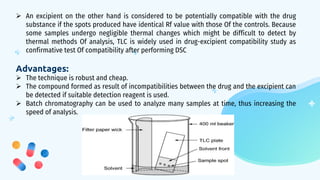
This document discusses drug-excipient interactions. It begins by introducing excipients and their important roles in drug formulations. It then describes common types of excipients and outlines four main types of drug-excipient interactions: physical, chemical, biopharmaceutical, and excipient-excipient. Several analytical techniques for detecting interactions are also discussed, including thermal methods like DSC and DTA, spectroscopy like FT-IR, and chromatography like TLC and HPLC. The document emphasizes the importance of studying drug-excipient compatibility to ensure formulation stability and safety.