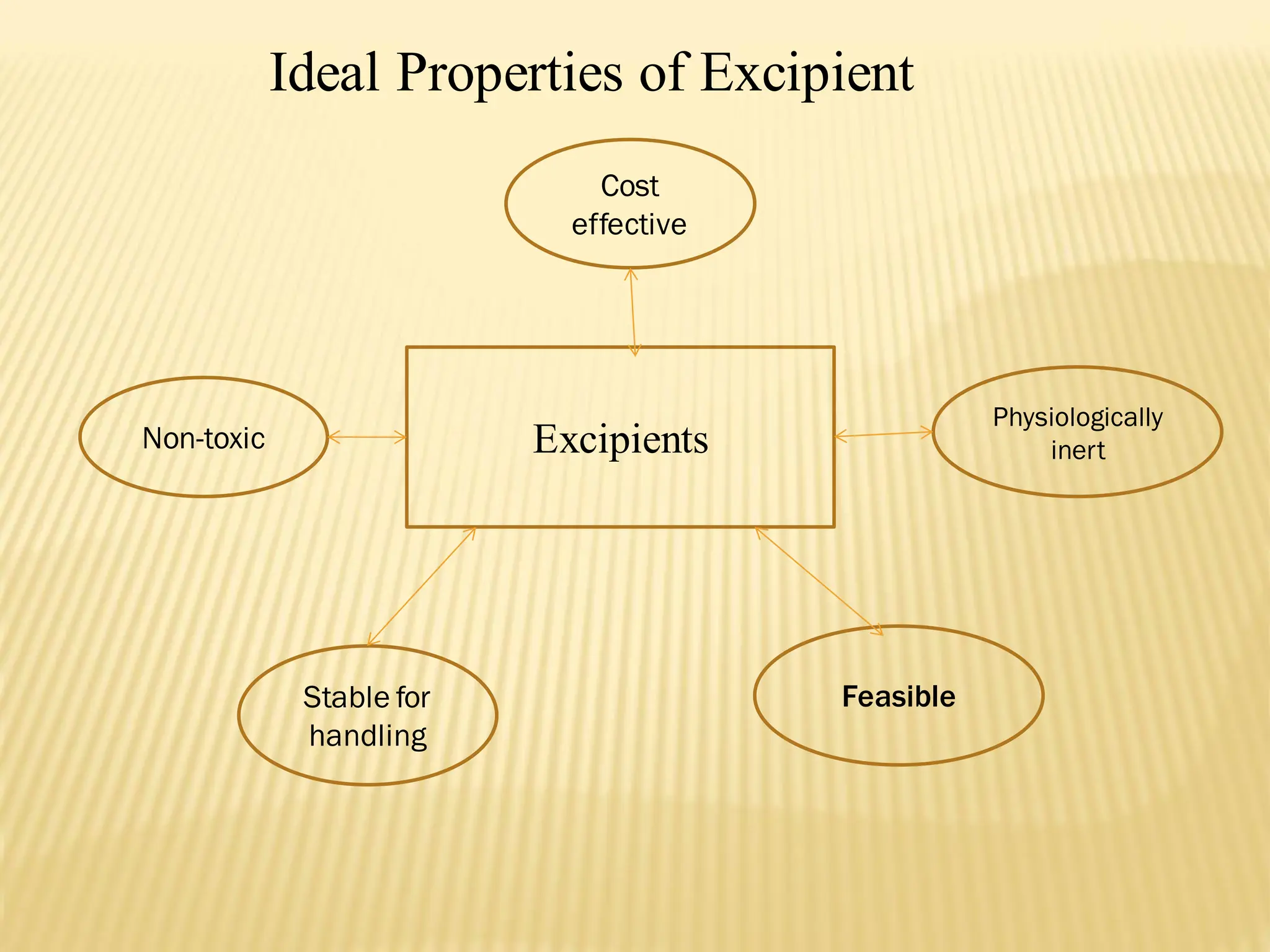
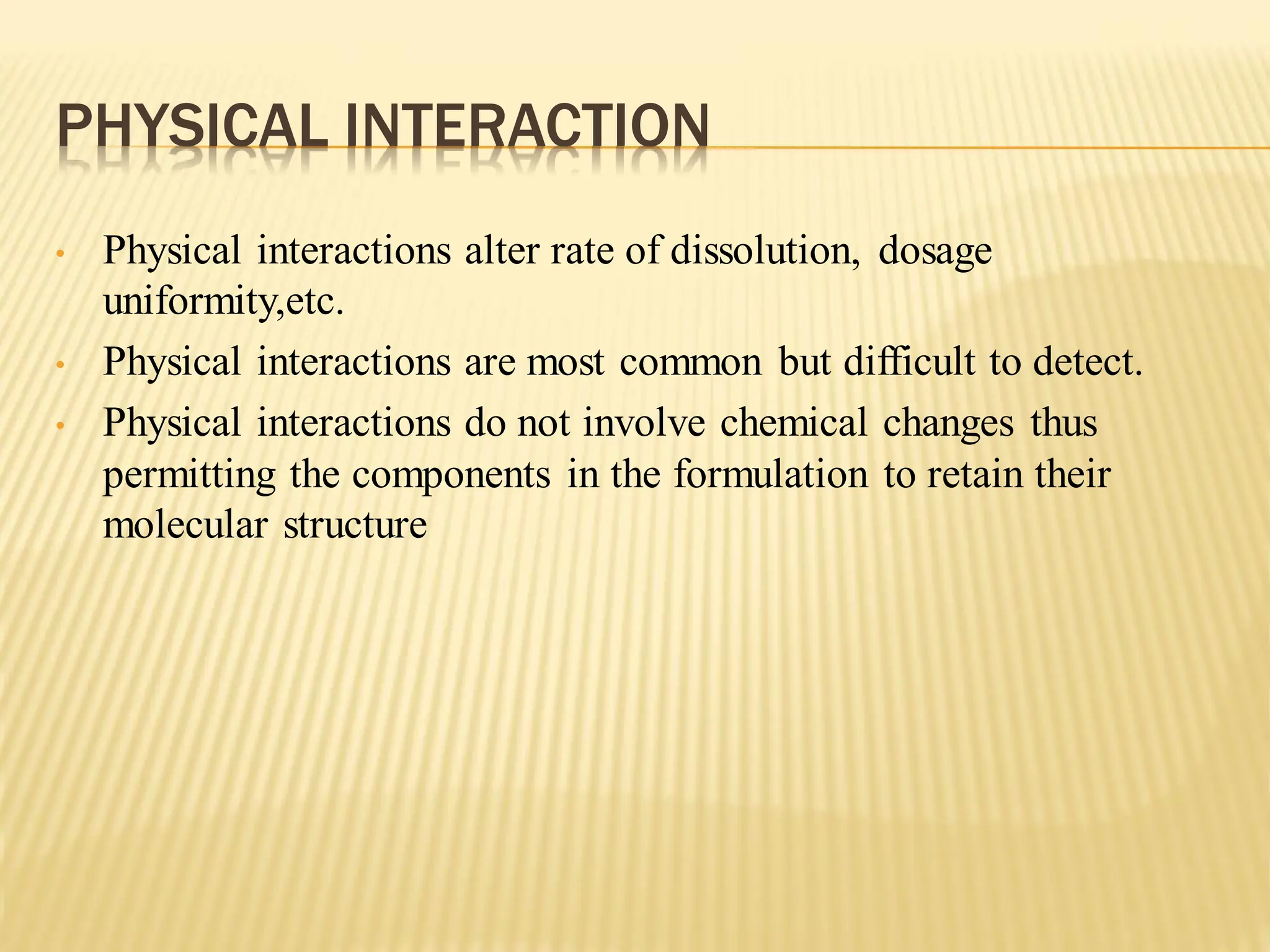
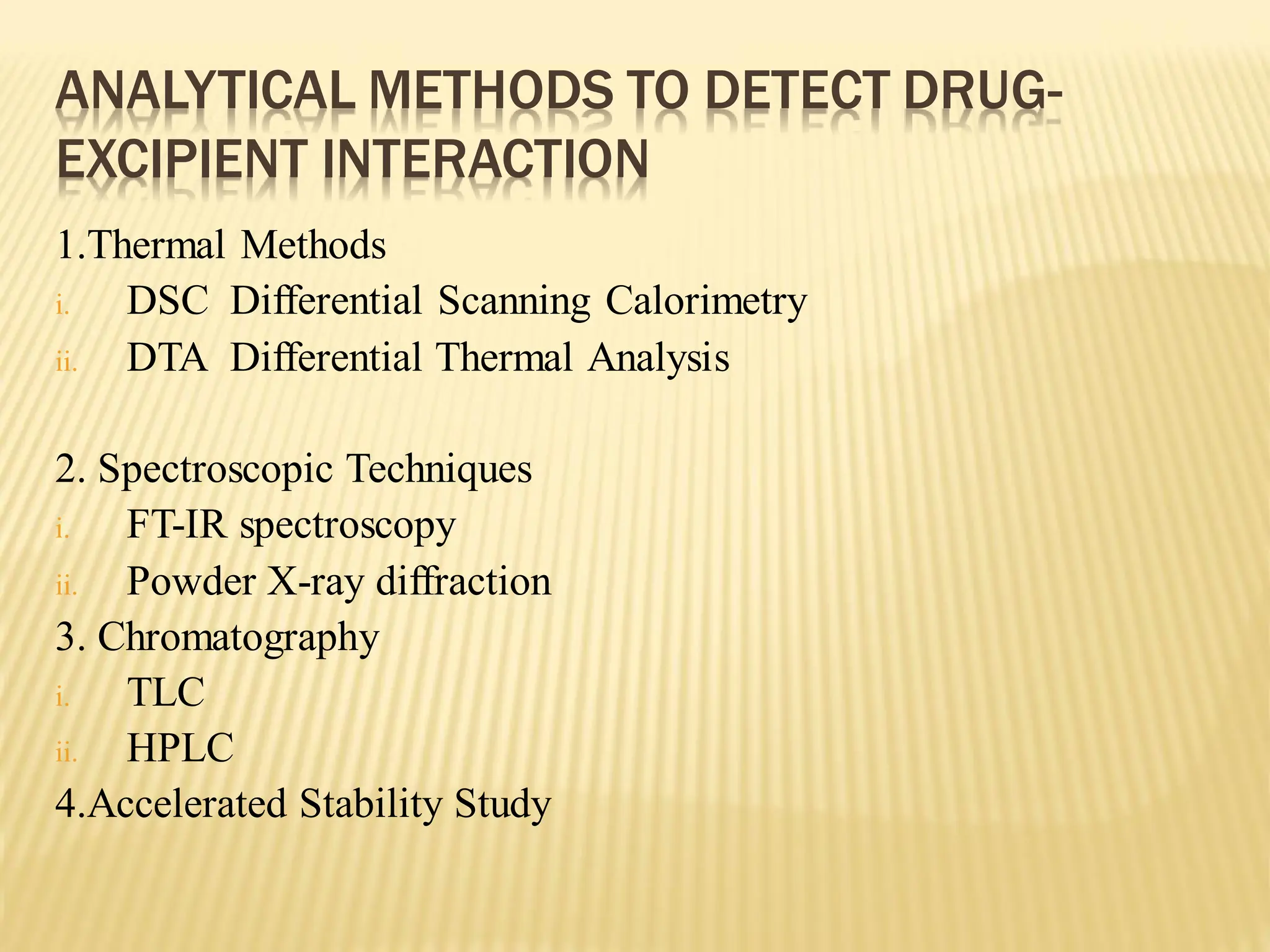
The document discusses a seminar on drug-excipient interactions and their detection methods, highlighting the importance of excipients in pharmaceutical formulations. It covers types of interactions, including physical, chemical, and biopharmaceutical, with examples of both beneficial and detrimental effects. Additionally, it outlines various analytical techniques used to detect these interactions.