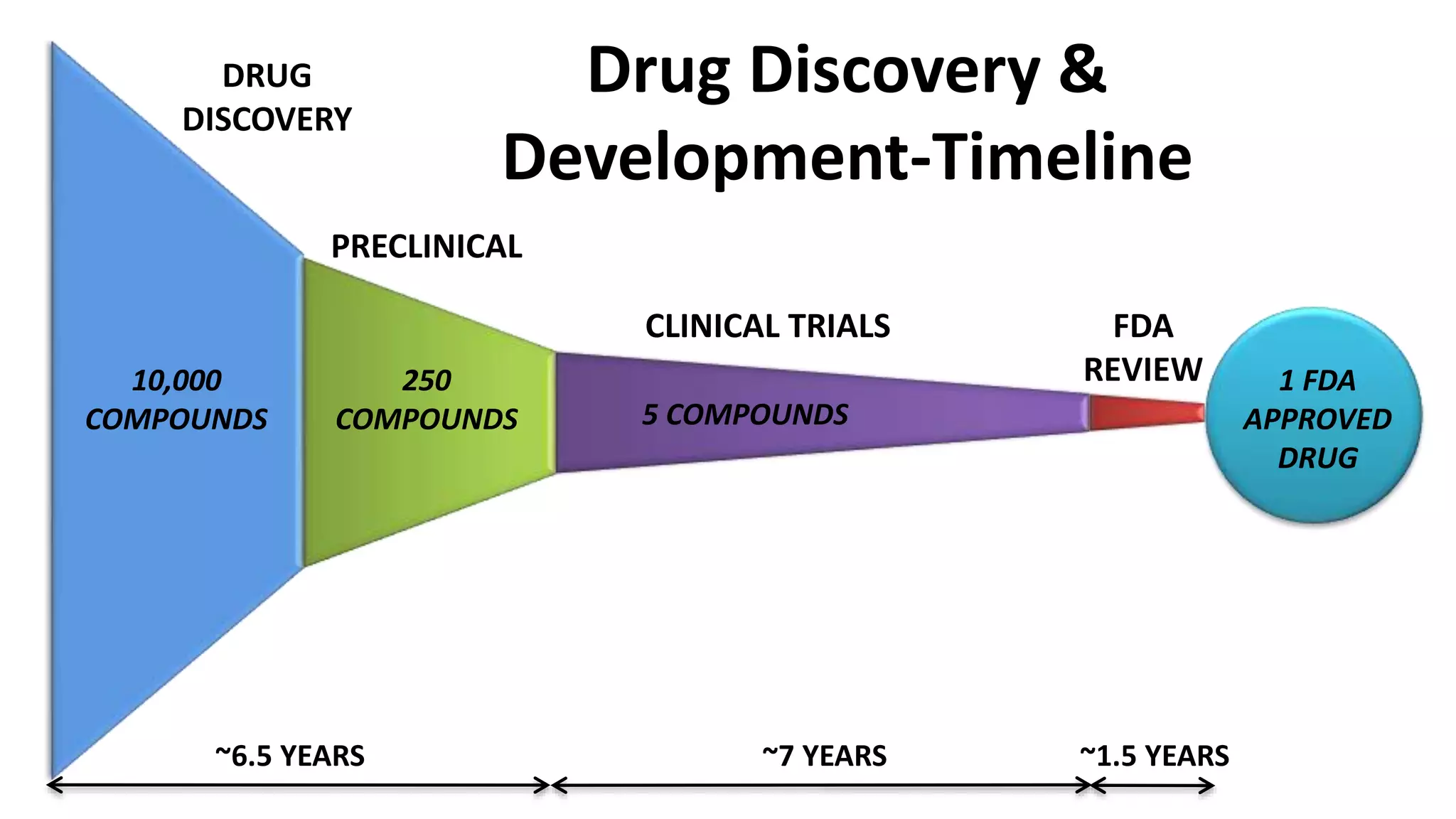
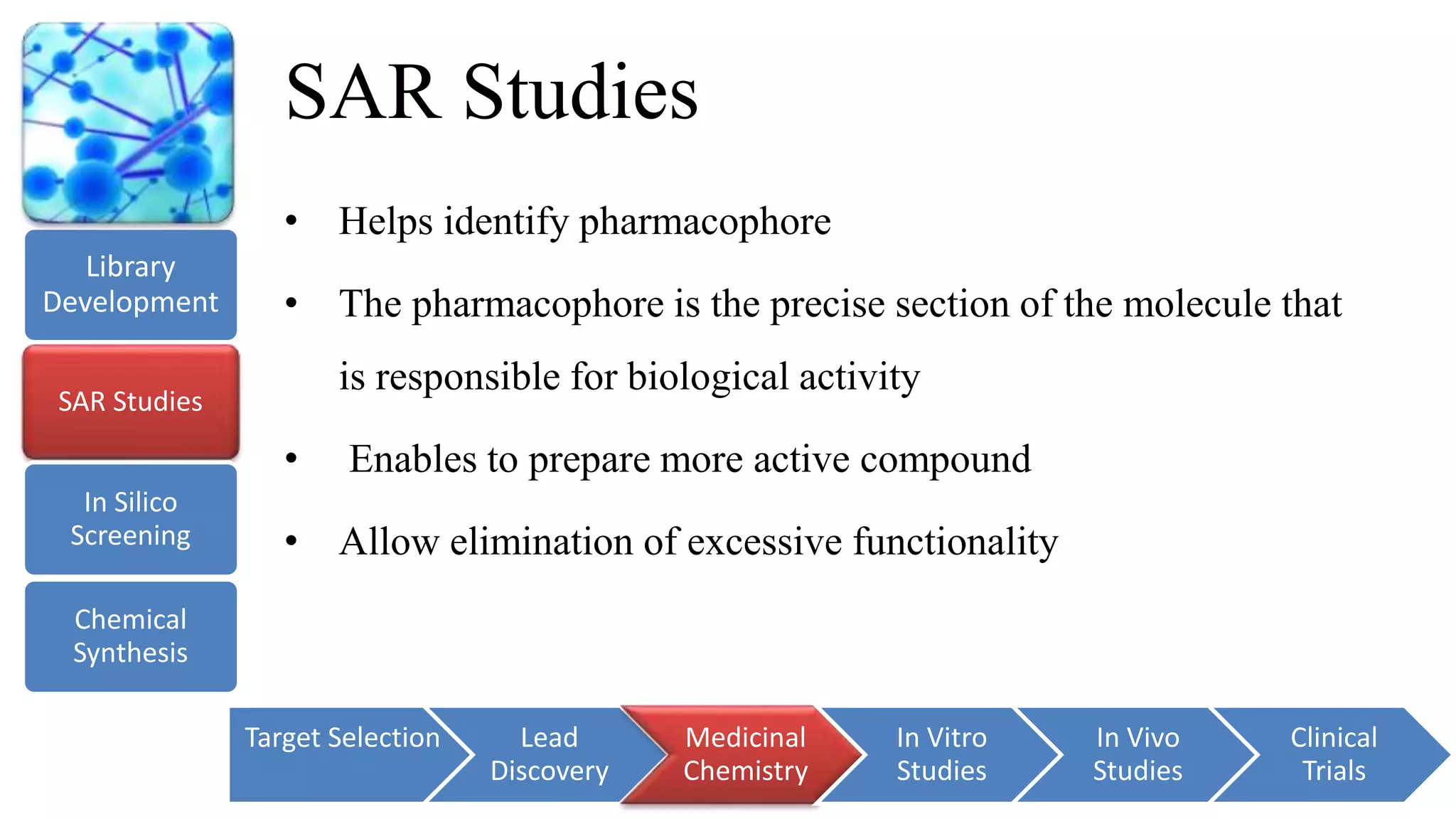
The document provides an overview of the drug discovery and development process. It discusses the various stages involved, including target selection using genomics, proteomics and bioinformatics; lead discovery through synthesis, isolation and high-throughput screening; medicinal chemistry such as structure-activity relationships studies; in vitro and preclinical in vivo testing in animal models; and clinical trials in humans. The timeline for this process can span over 10-15 years from drug target identification to regulatory approval. Key techniques and approaches at each stage are also summarized.