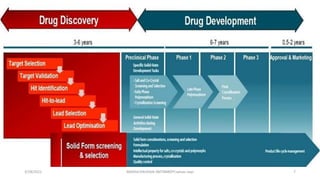
This document outlines the principles of drug discovery and development, detailing the historical context of drug discovery, particularly the transition from traditional herbal remedies to modern synthetic drugs and antibiotics. It describes the drug development process in three phases: discovery, development, and commercialization, emphasizing the importance of efficiency, regulatory approvals, and clinical trials. The document also highlights the complexities of clinical research, including design, execution, and aiming for a favorable benefit-to-risk ratio in various patient populations.