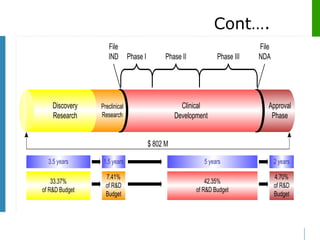
This document discusses various approaches to drug discovery and development. It begins by outlining historical approaches such as studying plants used in traditional medicine. It then describes modern approaches like studying disease processes and biological pathways. The document also discusses key events in the development of drugs like aspirin and quinine. It provides an overview of the drug development process, noting the high costs and failure rates. Various phases of clinical trials are defined. Pre-clinical development and requirements for an Investigational New Drug application to enter human trials are also summarized.