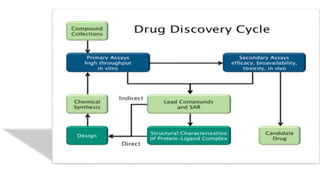
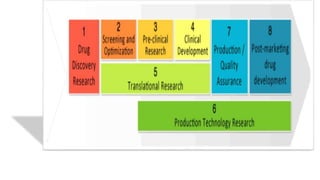
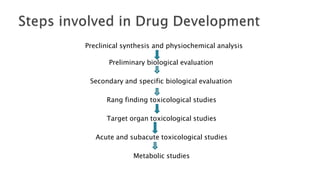
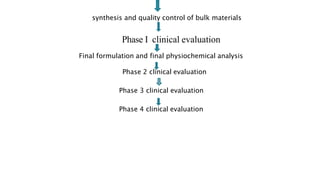
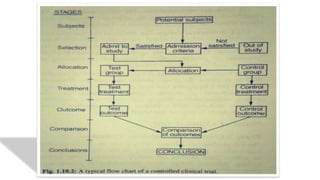
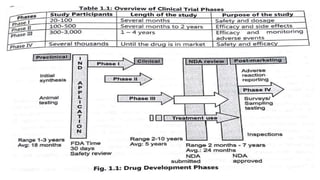
The document summarizes the stages of drug discovery and development. It begins with drug discovery, which involves understanding disease pathways and identifying drug targets. Lead compounds are then identified and optimized. Preclinical testing assesses safety. If successful, an investigational new drug application is filed and clinical trials proceed in four phases, from initial safety testing to large efficacy trials. If approved, post-marketing monitoring continues to assess long-term safety. The process aims to bring safe and effective therapies to patients while adhering to regulatory standards.