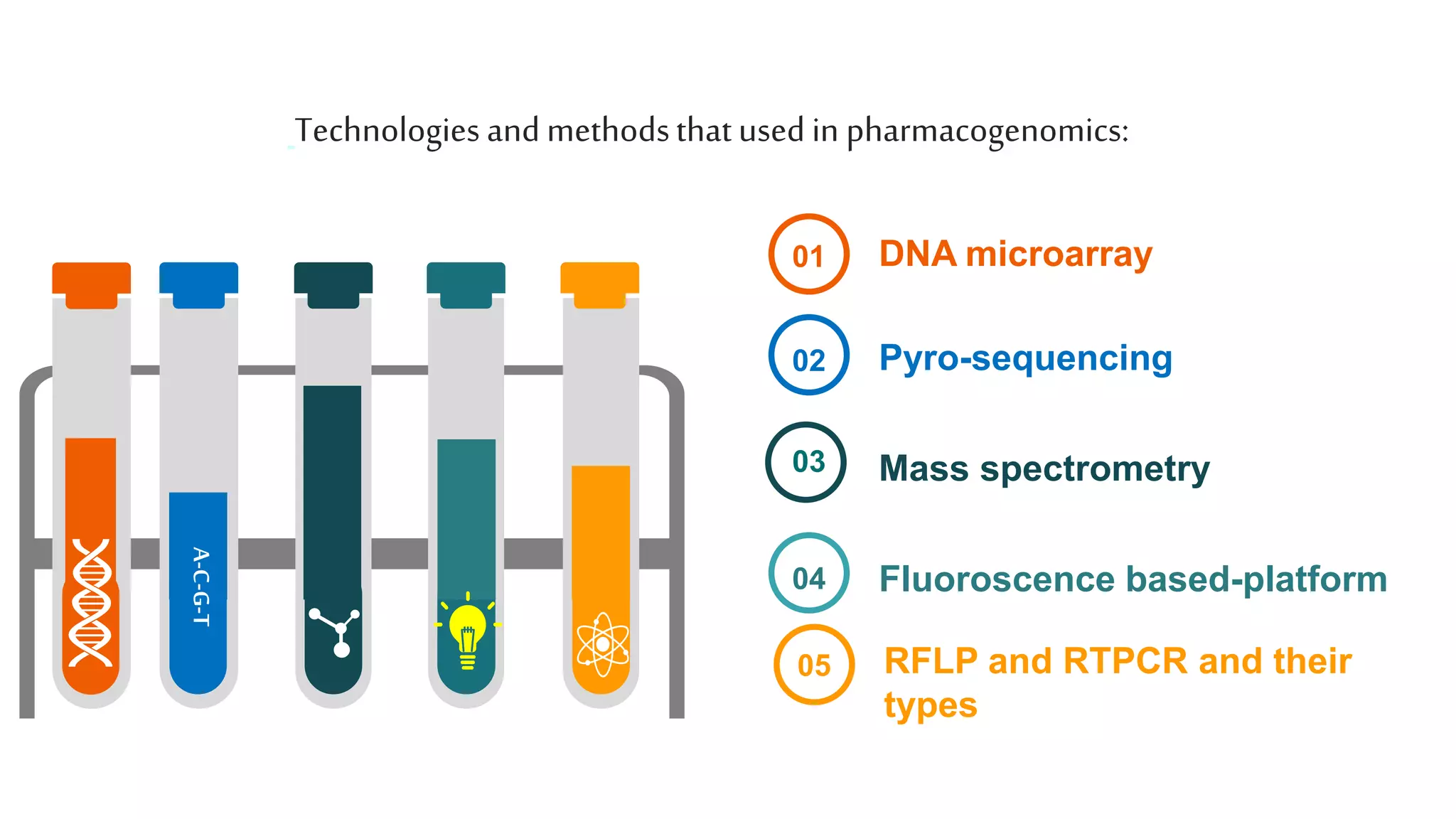
Pharmacogenomics aims to optimize drug therapy based on a patient's genotype to maximize efficacy and minimize adverse effects. It involves studying how genetic factors influence individual responses to drugs in terms of absorption, distribution, metabolism, and excretion. Genetic polymorphisms like SNPs that occur in over 1% of the population can impact a drug's effects. Pharmacogenomic testing identifies biomarkers related to drug metabolism and targets to determine effective treatments and dosages for patients. While it holds promise for improving drug development and personalized medicine, limitations include insufficient validation and high costs.