This document discusses disease screening and provides information on various aspects of screening programs and tests. It defines screening as actively searching for unrecognized disease in apparently healthy individuals using simple tests. The key points are:
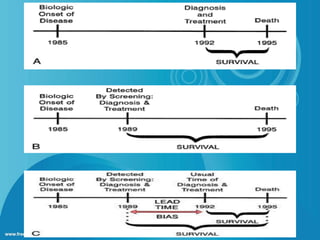
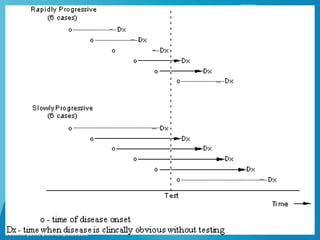
- Screening is part of secondary prevention and aims to detect diseases early when they may be still curable. It involves testing populations, not individuals with symptoms.
- An ideal screening test is both highly sensitive and specific, but in practice these factors typically have an inverse relationship. Sensitivity and specificity can be adjusted by changing the test cutoff criteria.
- For a screening program to be effective, the disease must be an important health problem that can be detected early and treated effectively to improve outcomes. The screening test