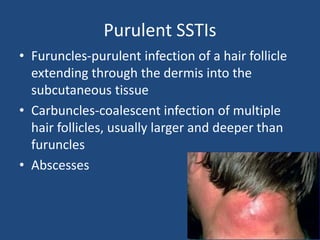
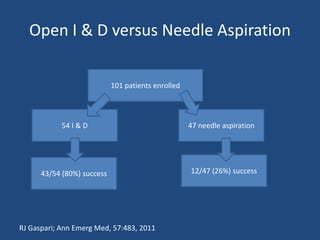
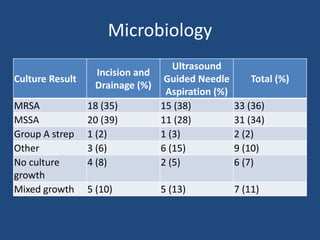
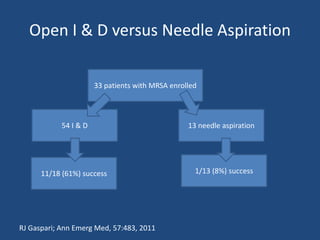
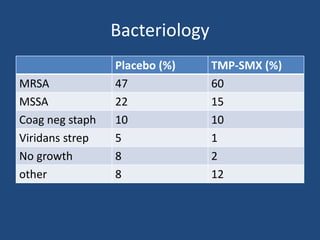
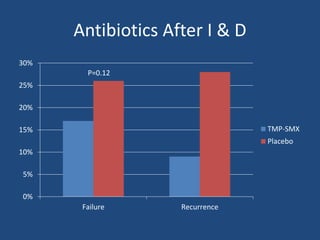
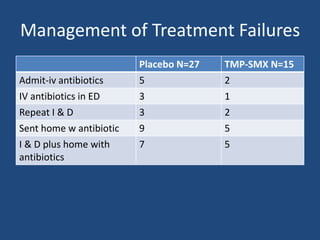
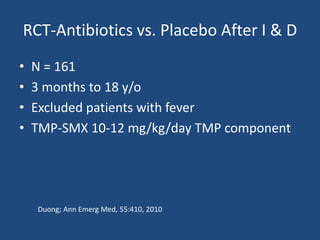
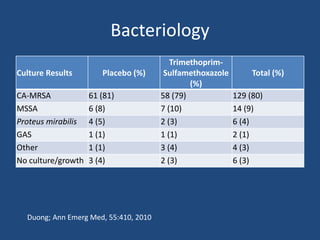
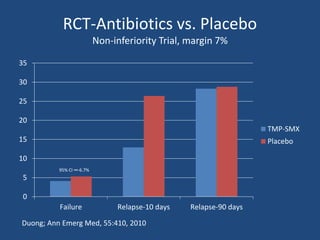
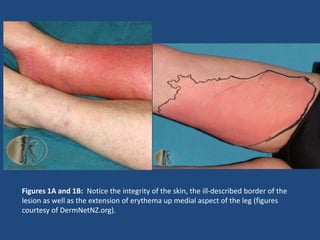
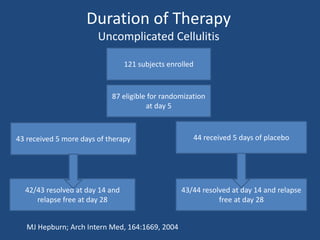
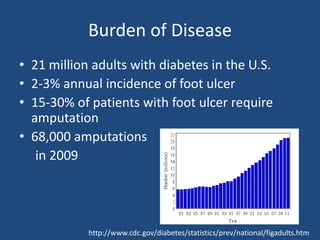
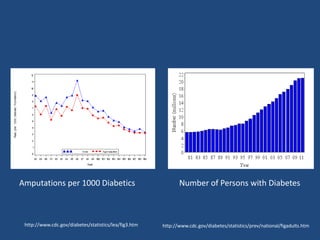
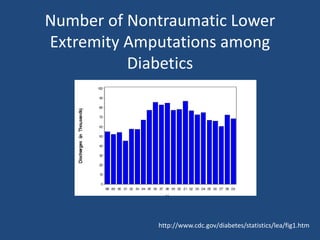
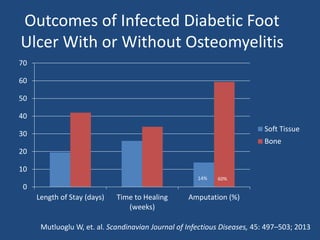
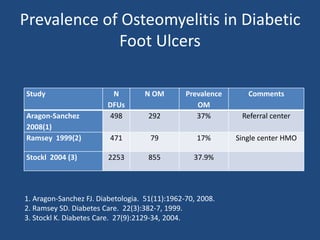
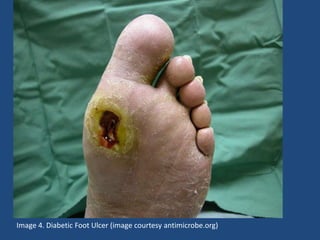
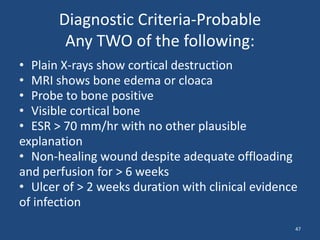
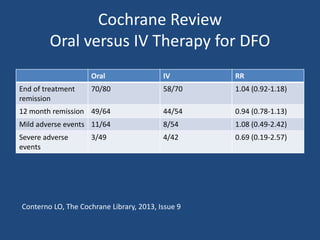
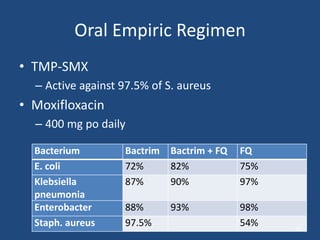
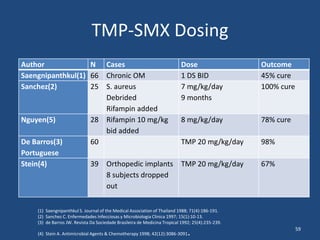
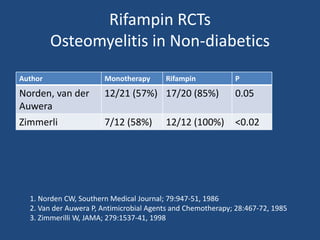
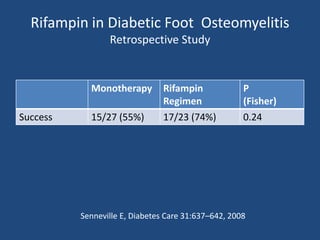
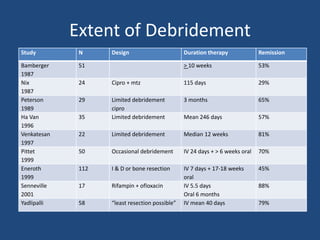
The document addresses the management and diagnosis of diabetic foot osteomyelitis, emphasizing the importance of recognizing at-risk patients and appropriate antibiotic use. It outlines diagnostic criteria for osteomyelitis and recommends empirical treatment protocols, including the use of broad-spectrum antibiotics and culture-directed therapy. Additionally, the document discusses the implications of diabetic foot infections and outlines the likelihood of amputation associated with osteomyelitis in diabetic patients.