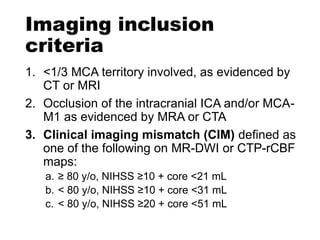
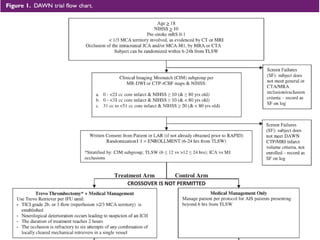
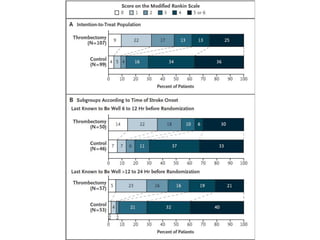
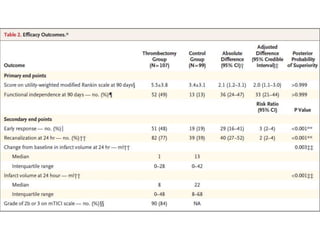
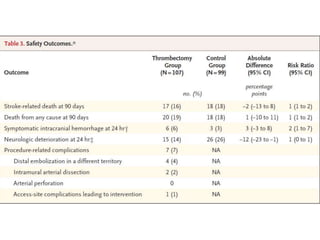
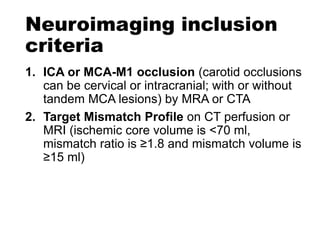
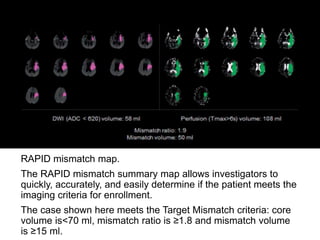
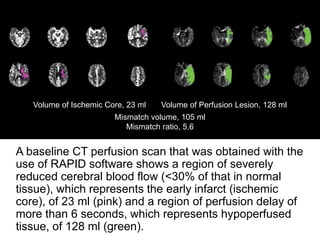
The document summarizes the inclusion criteria for two clinical trials: DAWN and DEFUSE 3. Both trials evaluated endovascular thrombectomy for acute ischemic stroke between 6-24 hours after onset. The general criteria for both trials were: age 18 or older, NIHSS score of 10 or higher, signs of acute ischemic stroke, and ability to receive treatment within 6-24 hours. The imaging criteria for both trials required an occlusion of the intracranial ICA or MCA and a clinical-imaging mismatch between the ischemic core and hypoperfused tissue on MRI/CT perfusion. DEFUSE 3 specifically required a core of less than 70ml, mismatch ratio of 1.8 or higher, and mismatch volume of 15