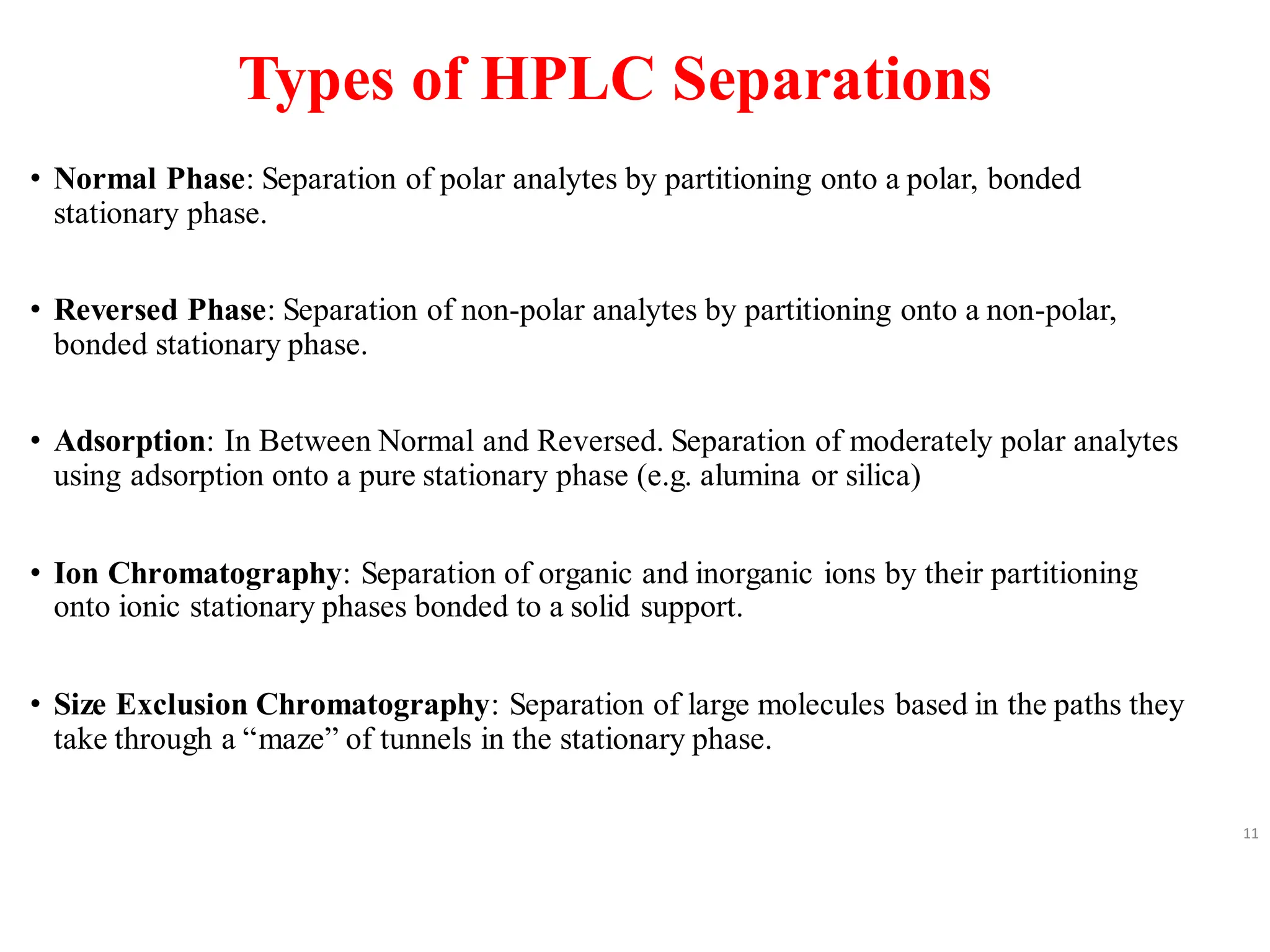
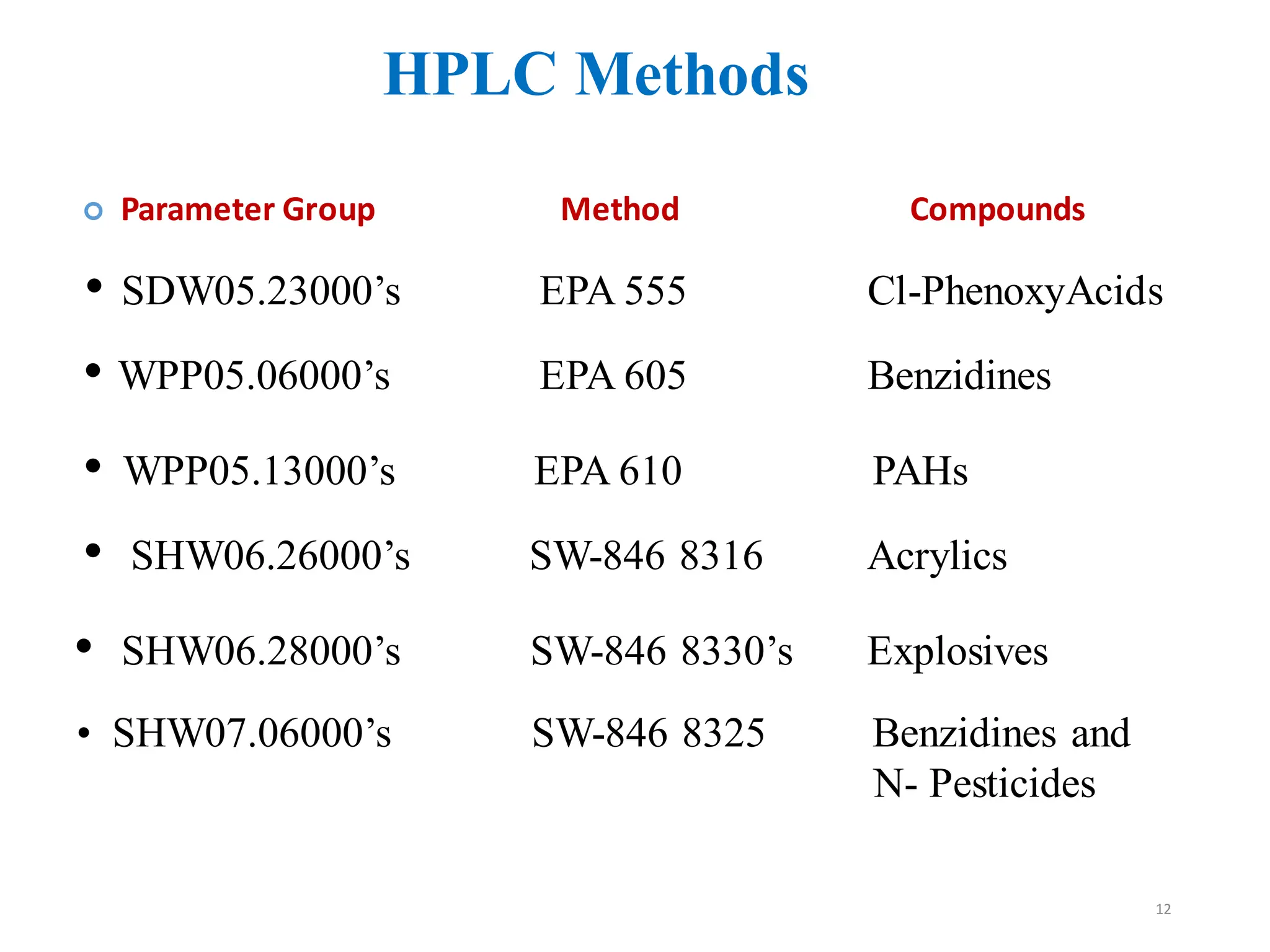
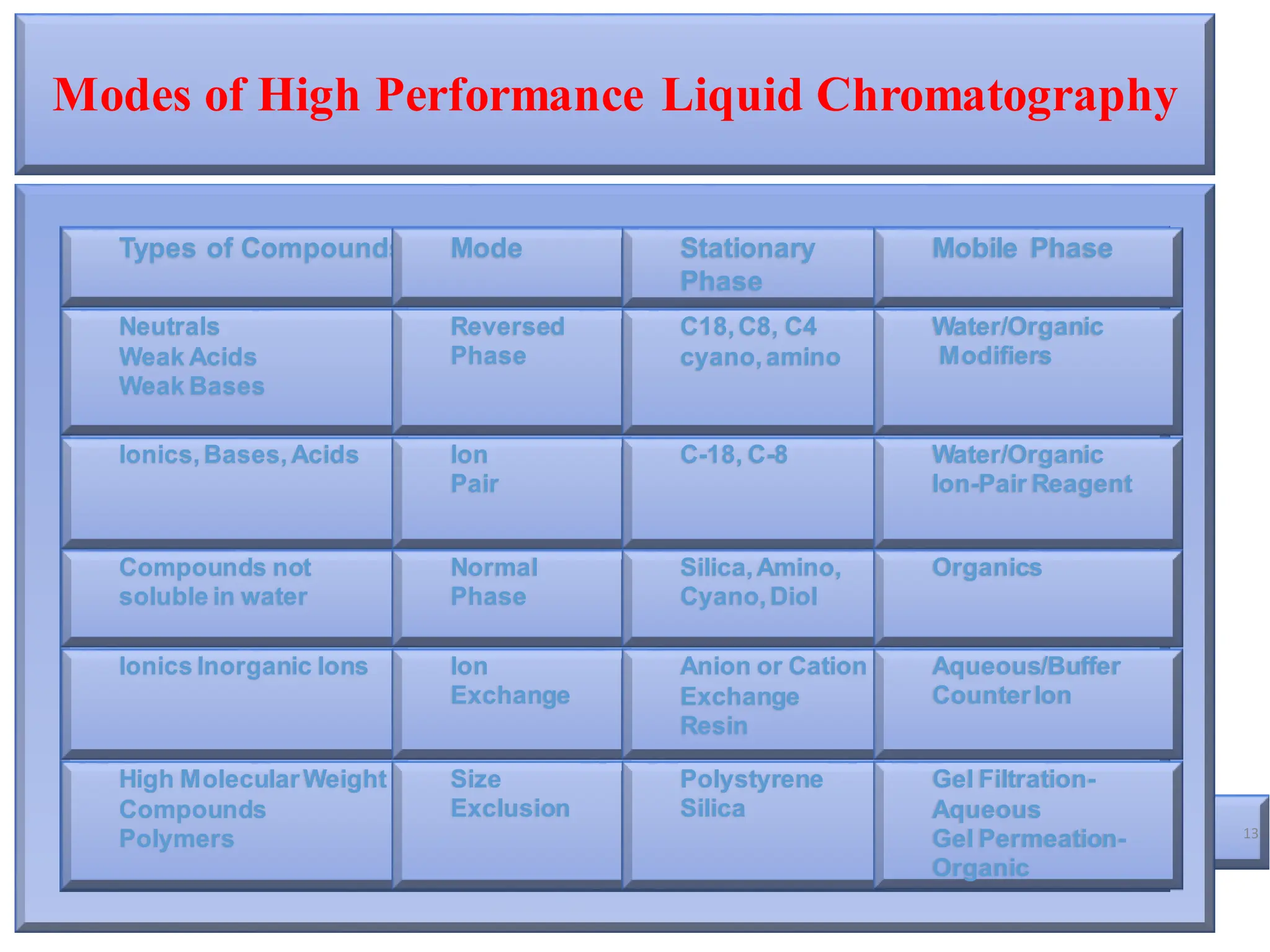
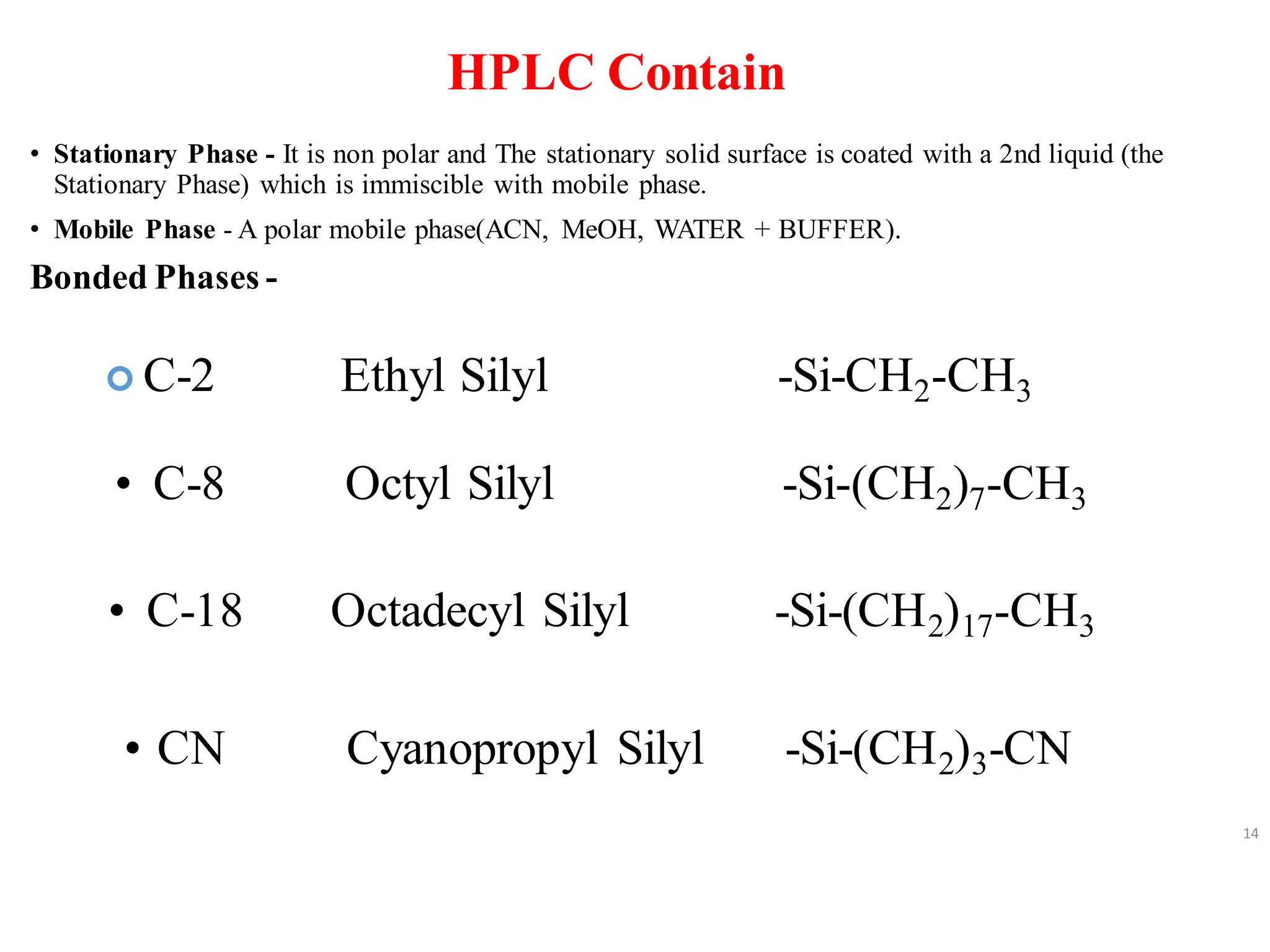
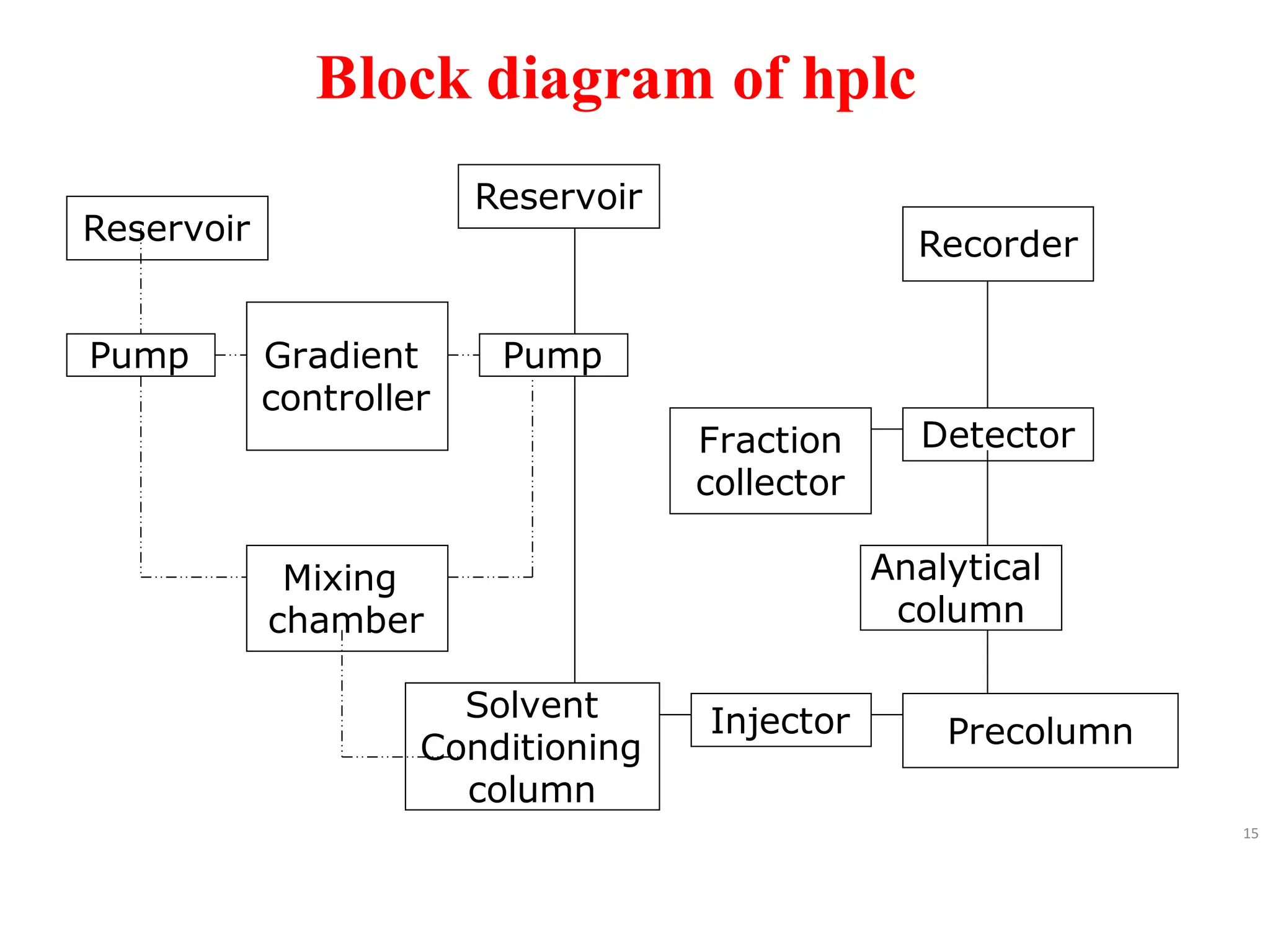
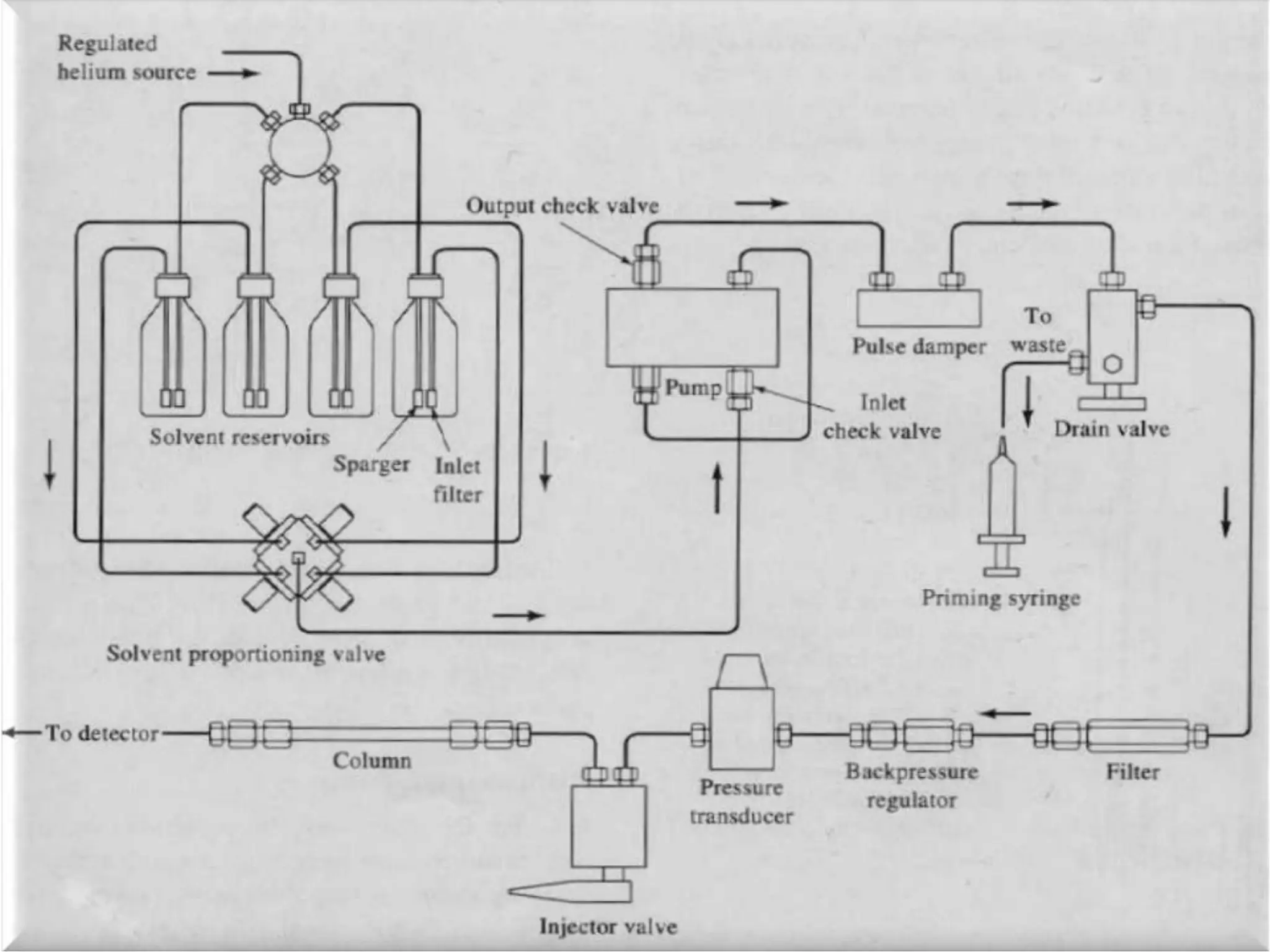
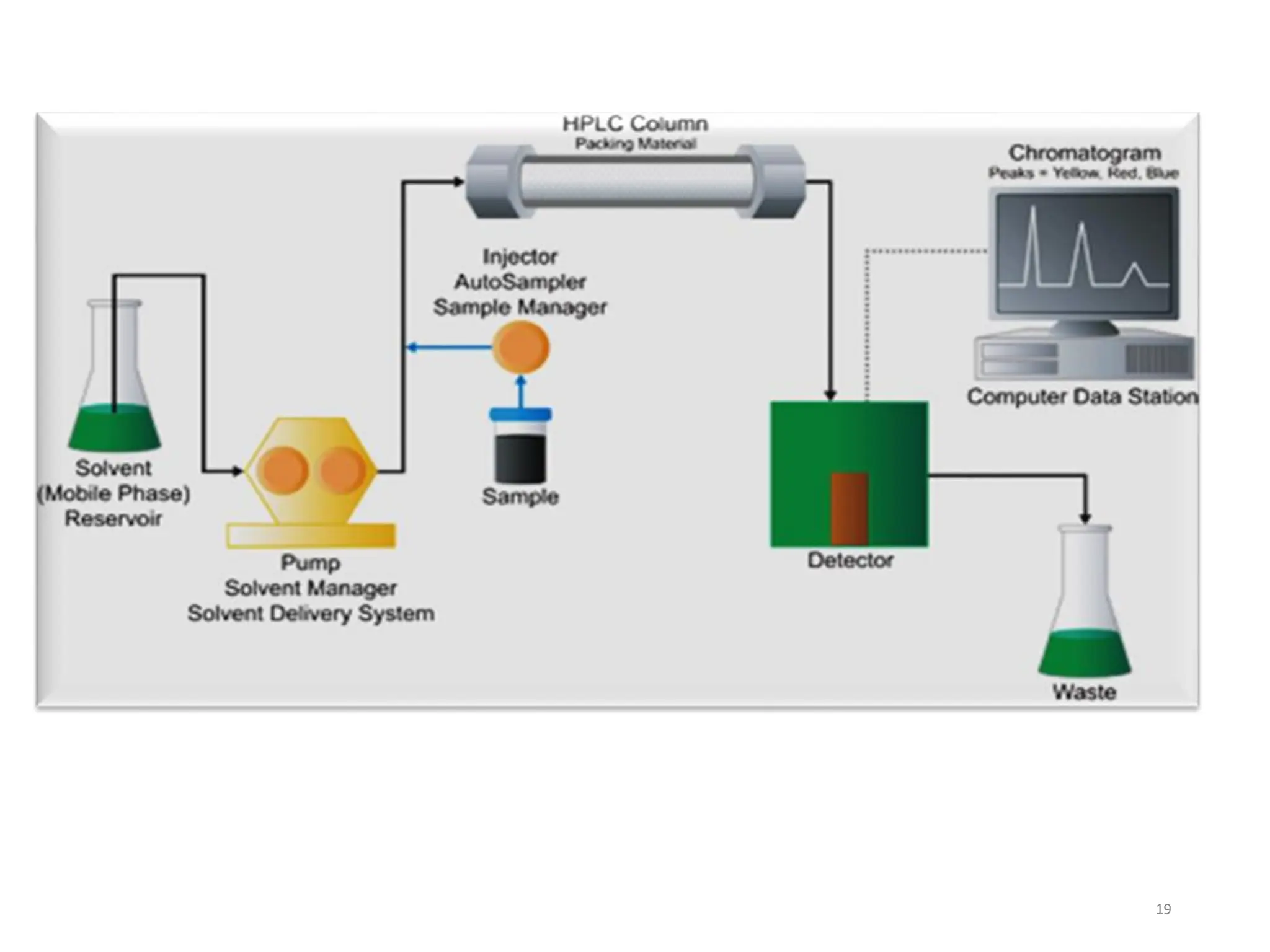
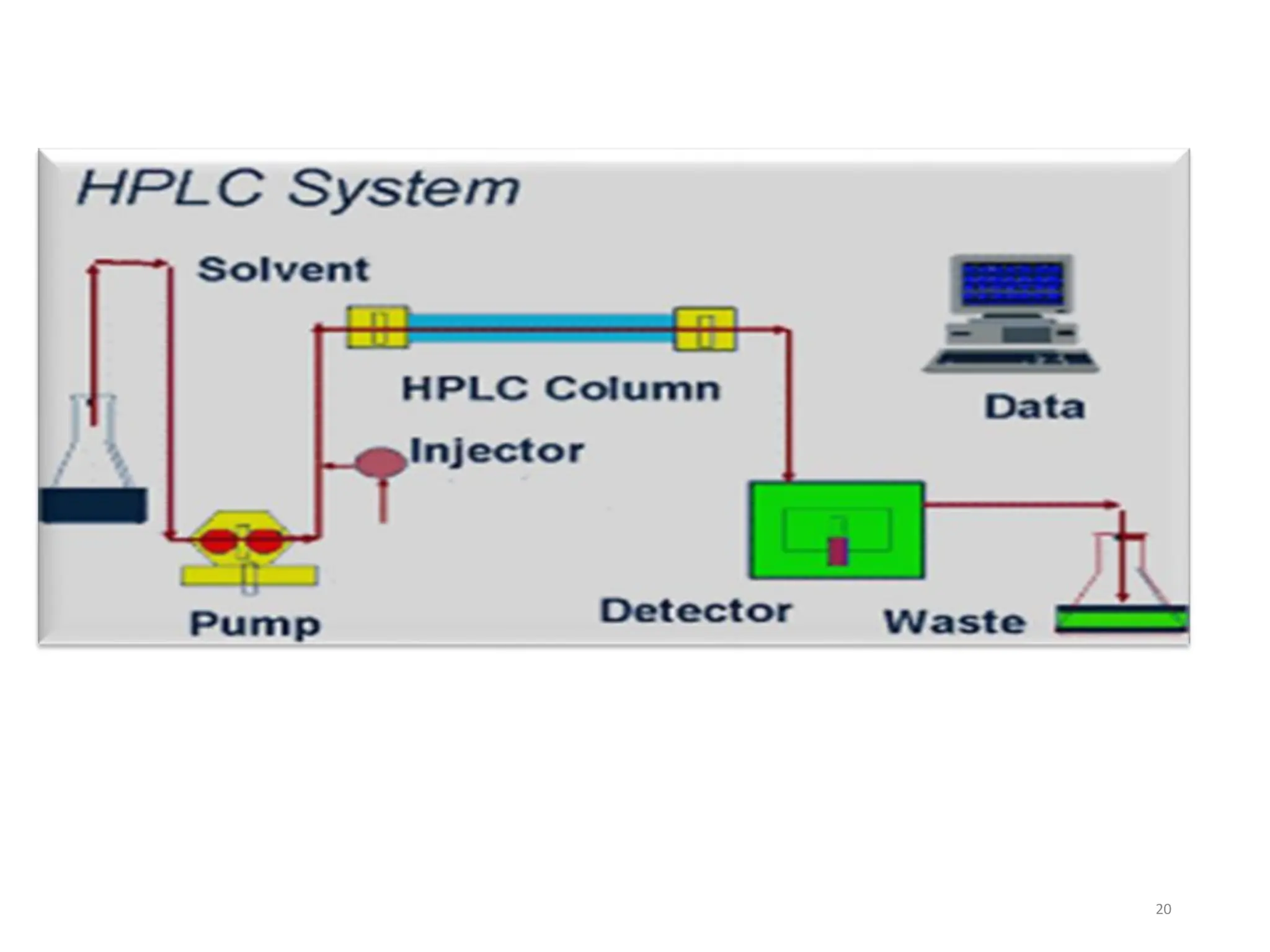
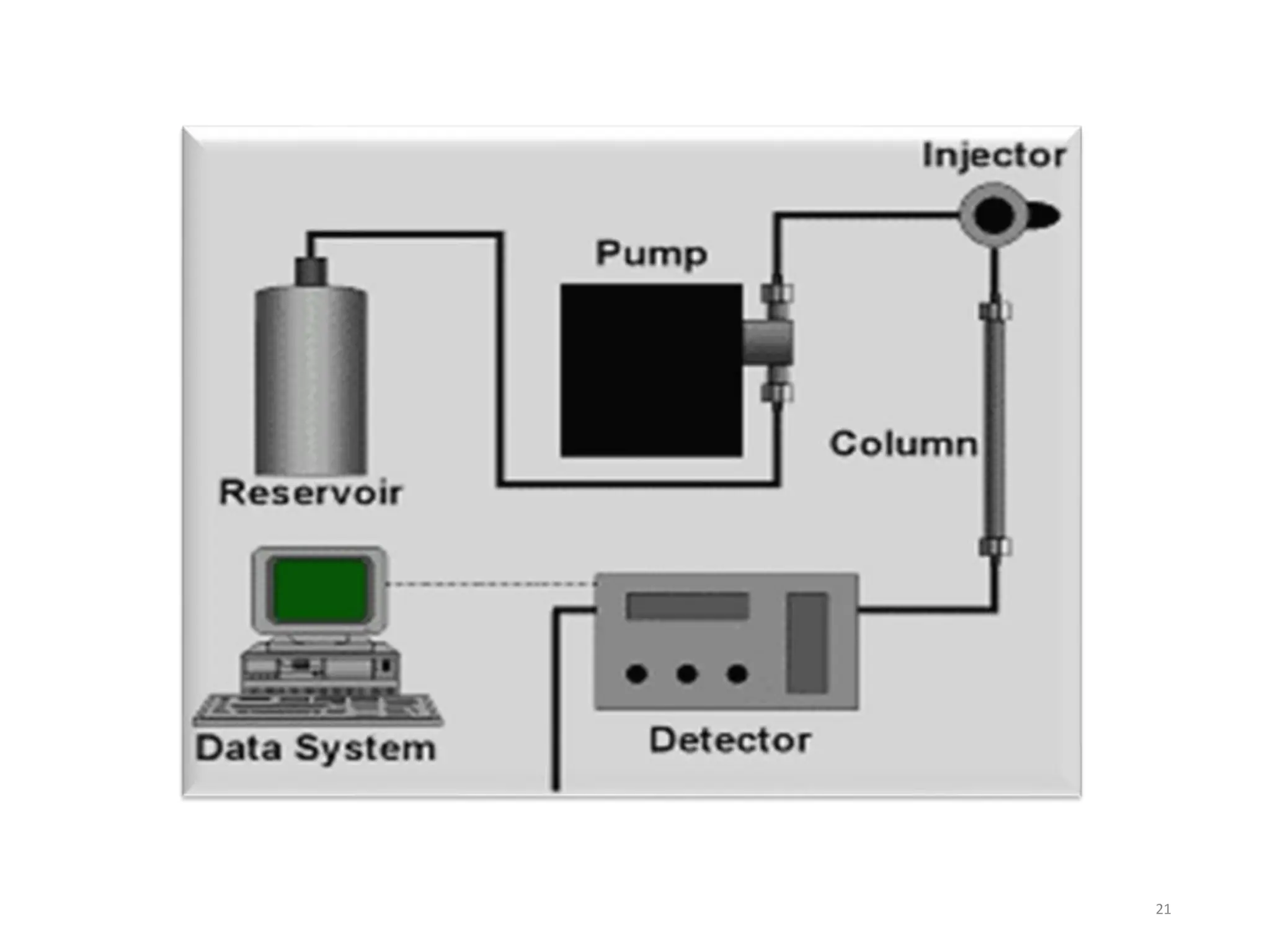
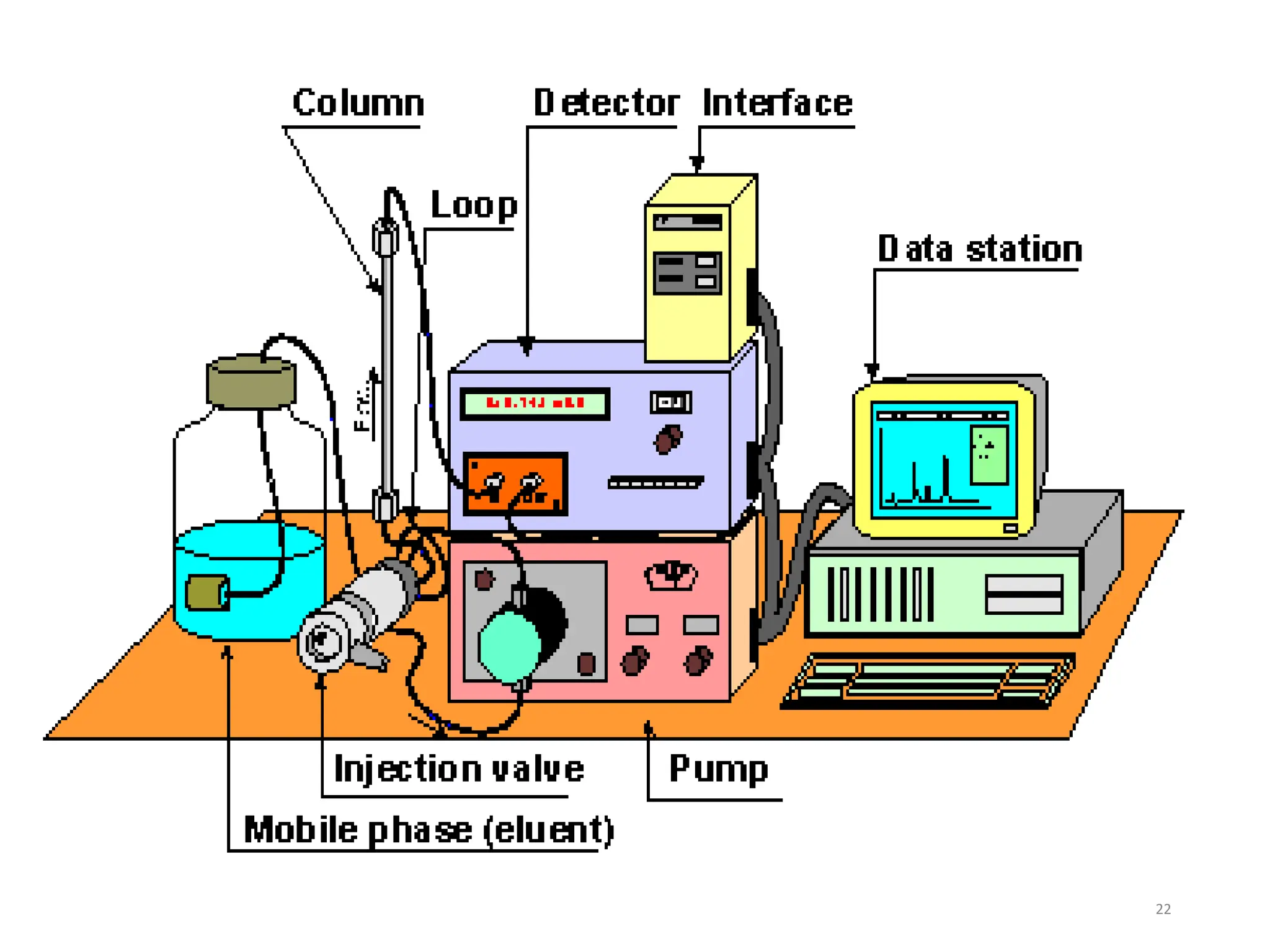
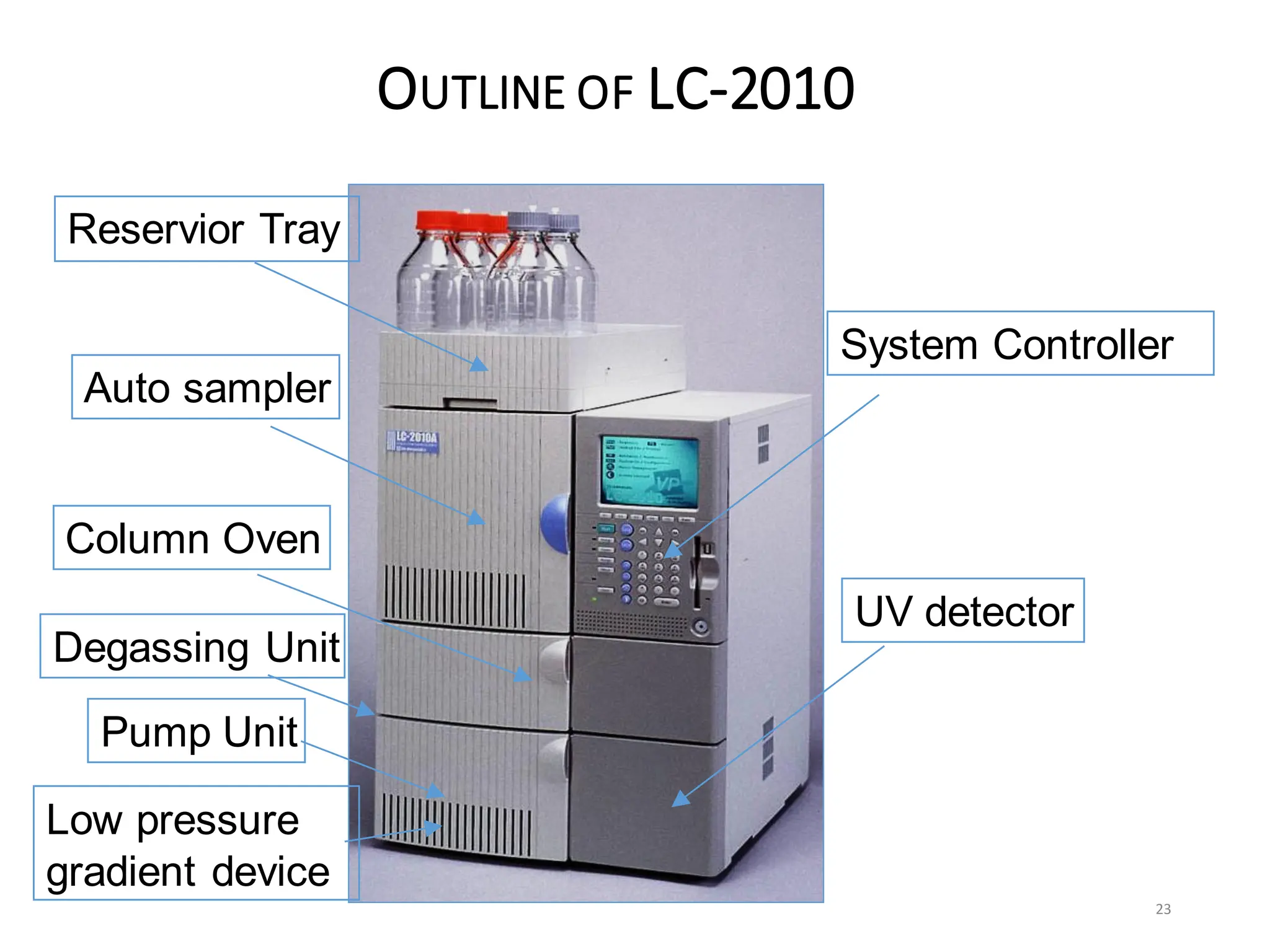
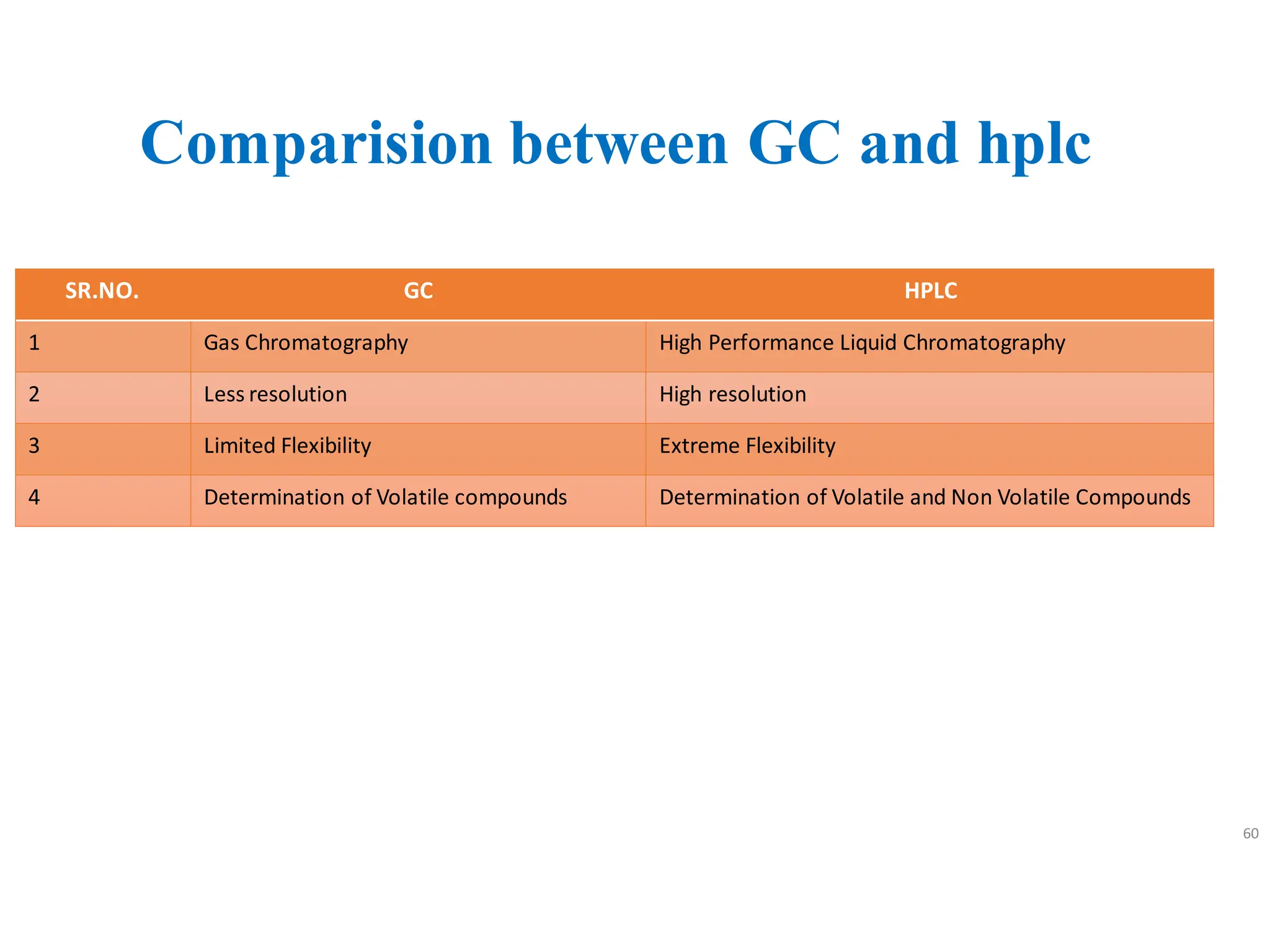
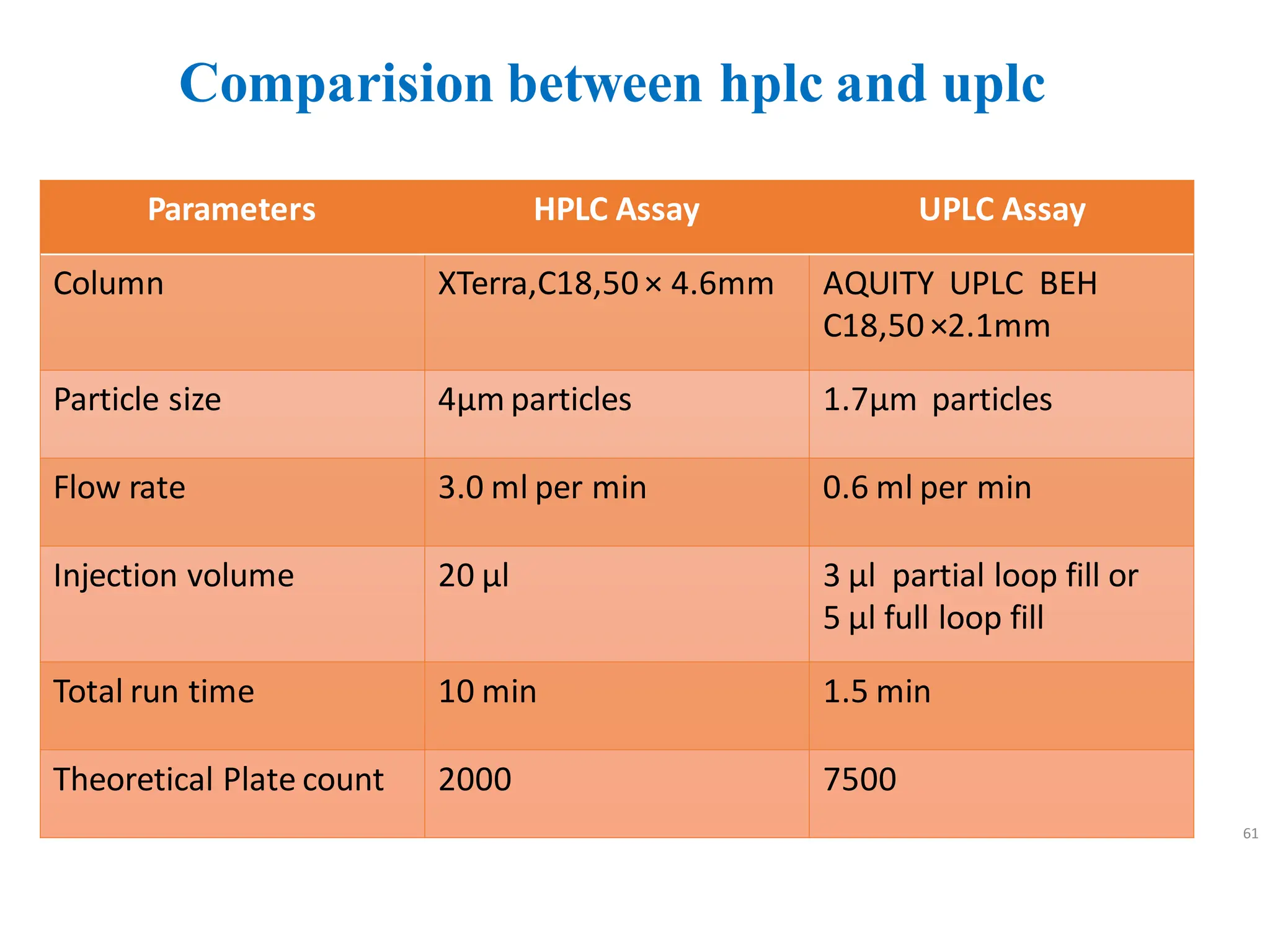
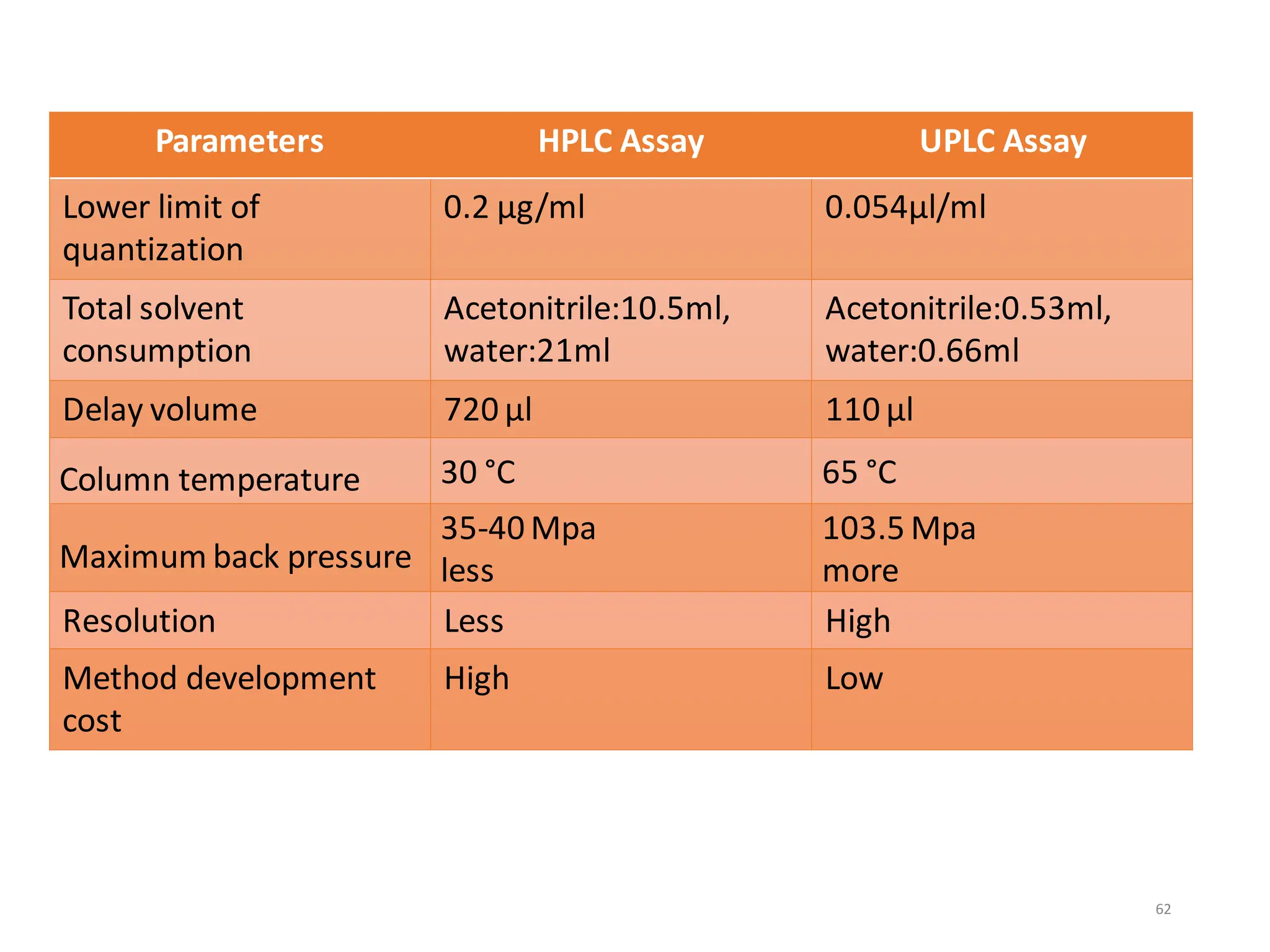
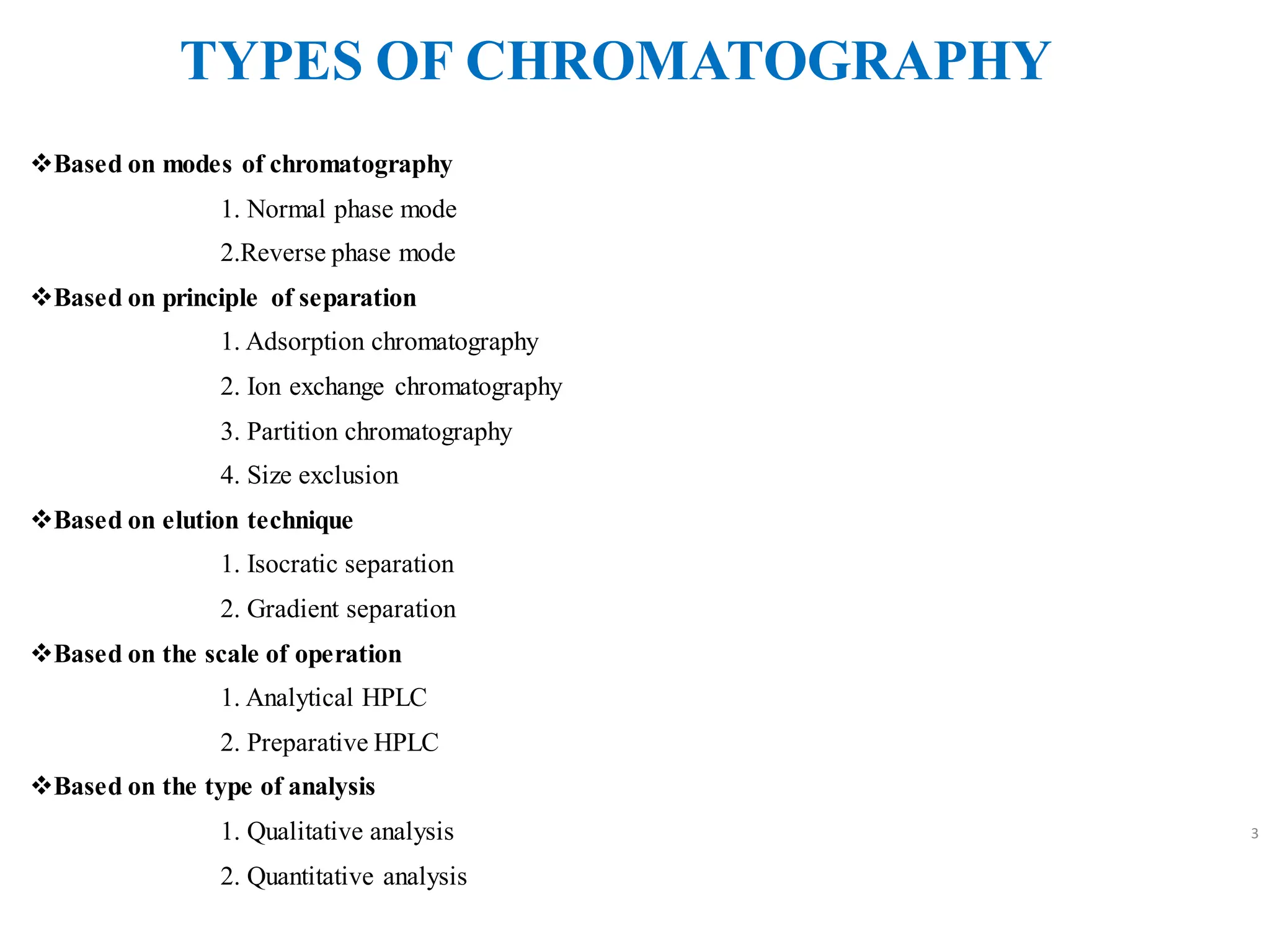
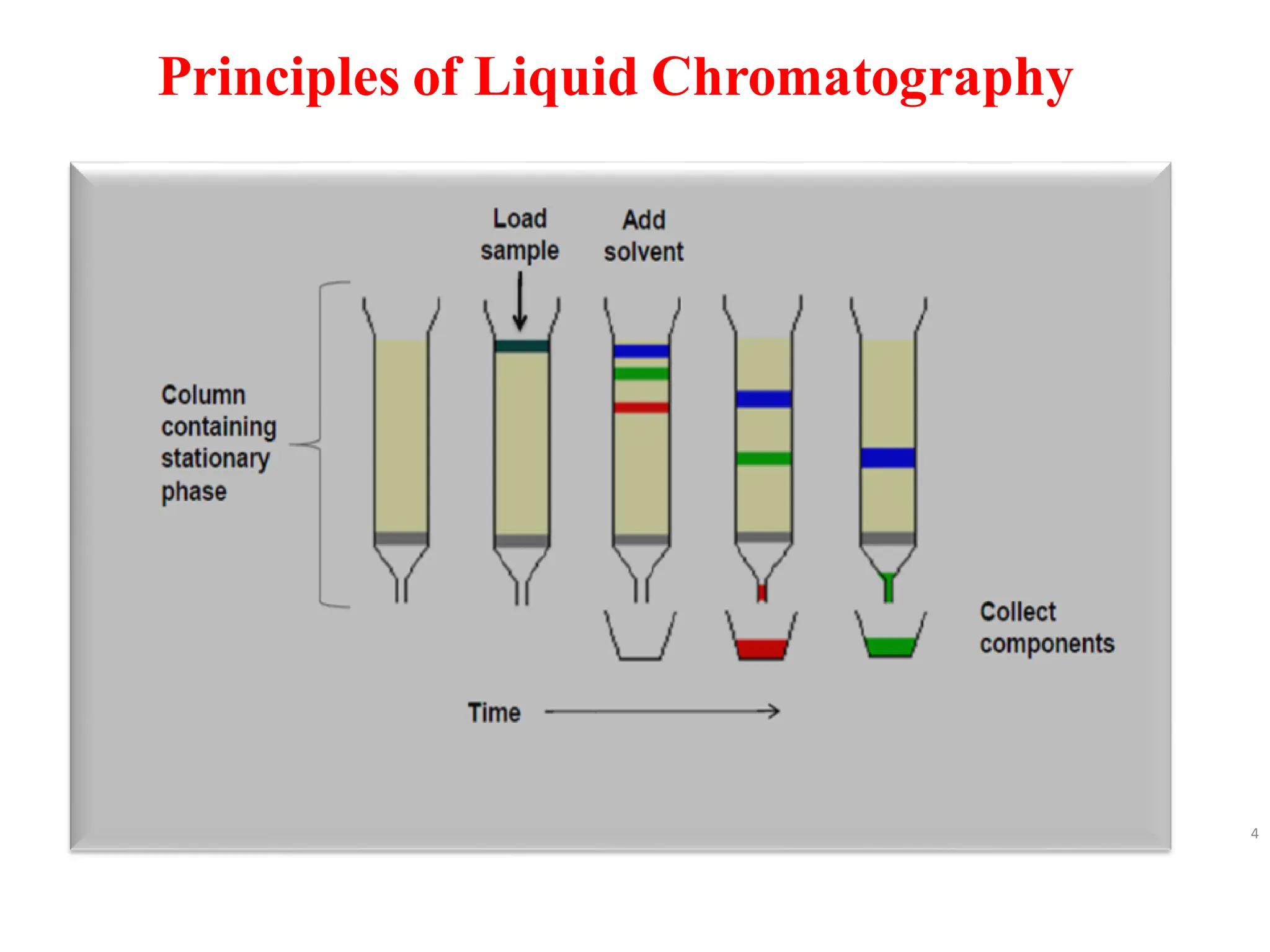
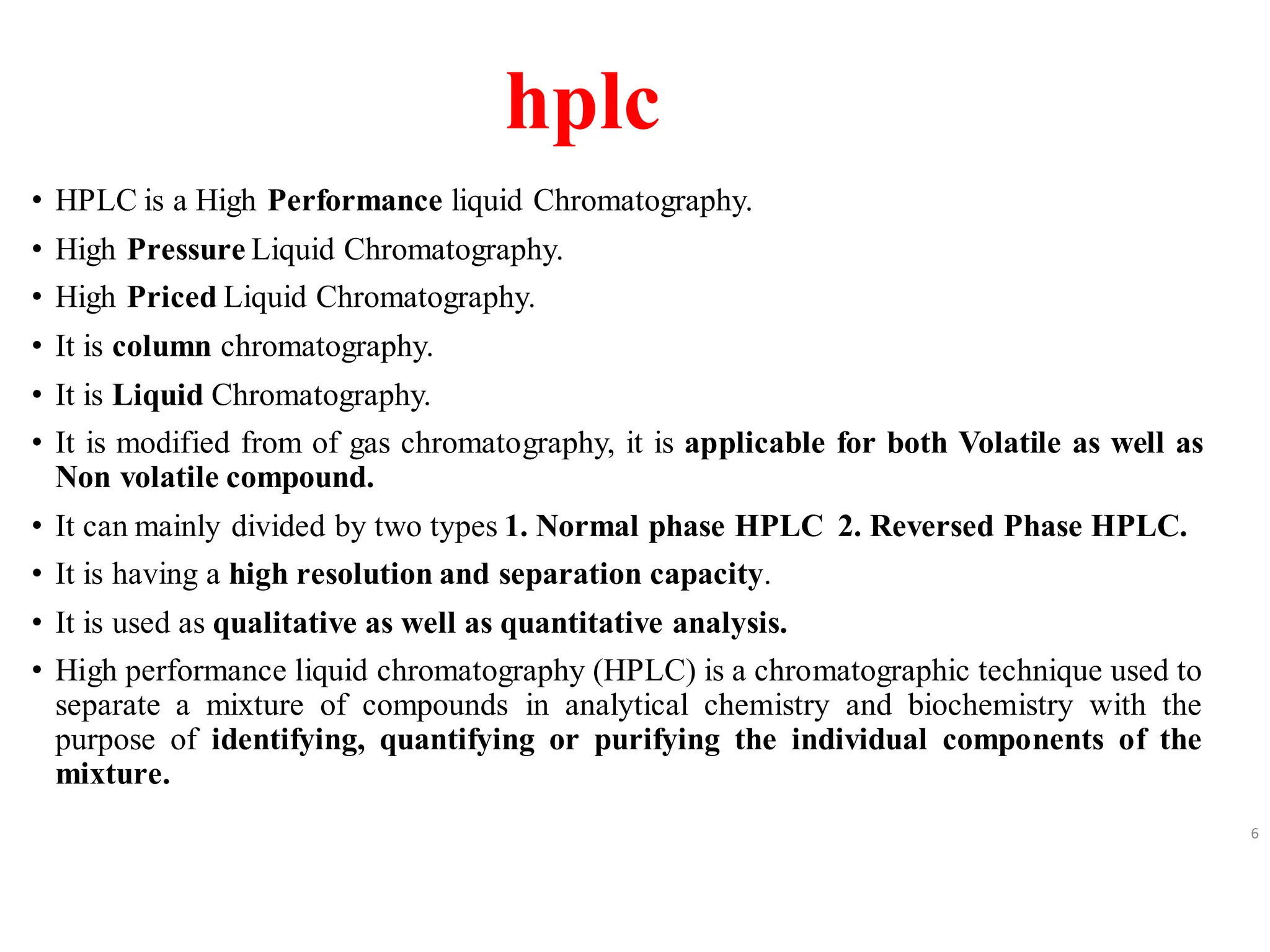
High Performance Liquid Chromatography (HPLC) is an analytical technique used to separate, identify, and quantify components in complex mixtures using a stationary and a mobile phase. Various modes of chromatography exist, such as normal phase and reversed phase, along with different elution techniques and types of detectors. HPLC is favored for its high sensitivity, rapid analysis, and good reproducibility, making it essential in fields like chemistry and biochemistry.
![High performance liquid
chromatography [HPLC]
1](https://image.slidesharecdn.com/hplc-241225135806-f1bf49c9/75/HIGH-PERFORMANCE-LIQUID-CHROMATOGRAPHY-HPLC-1-2048.jpg)

![Principle
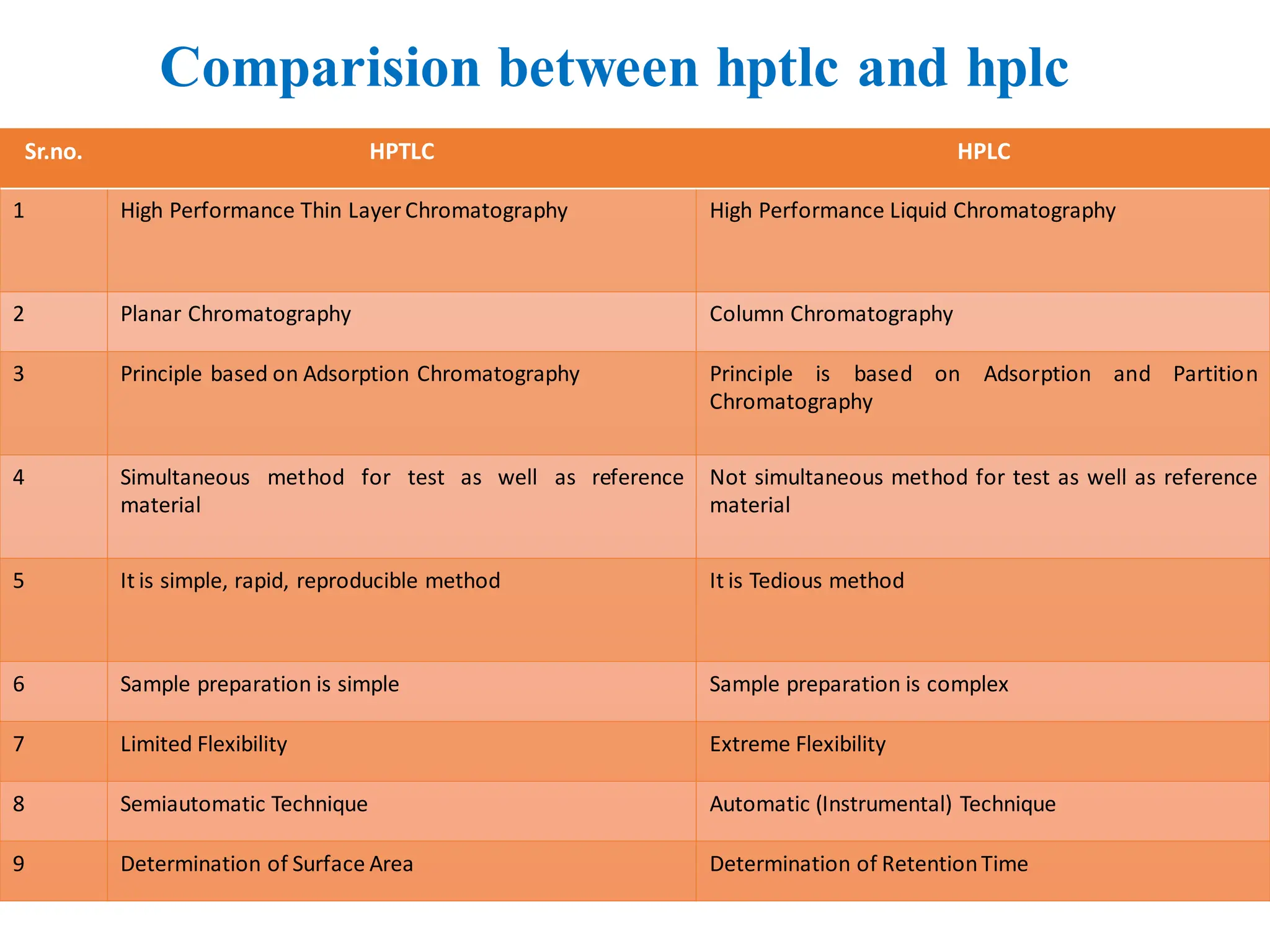
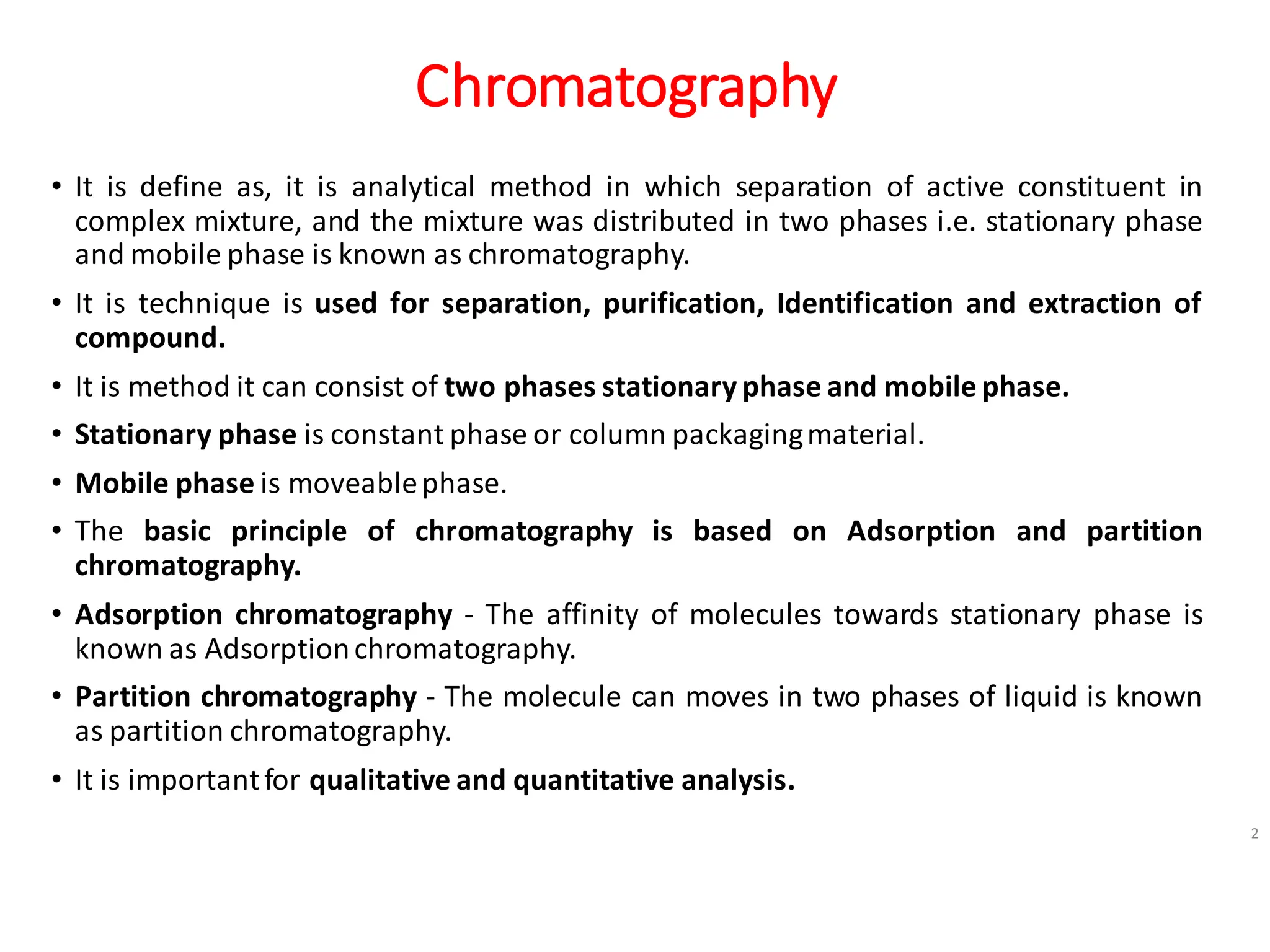
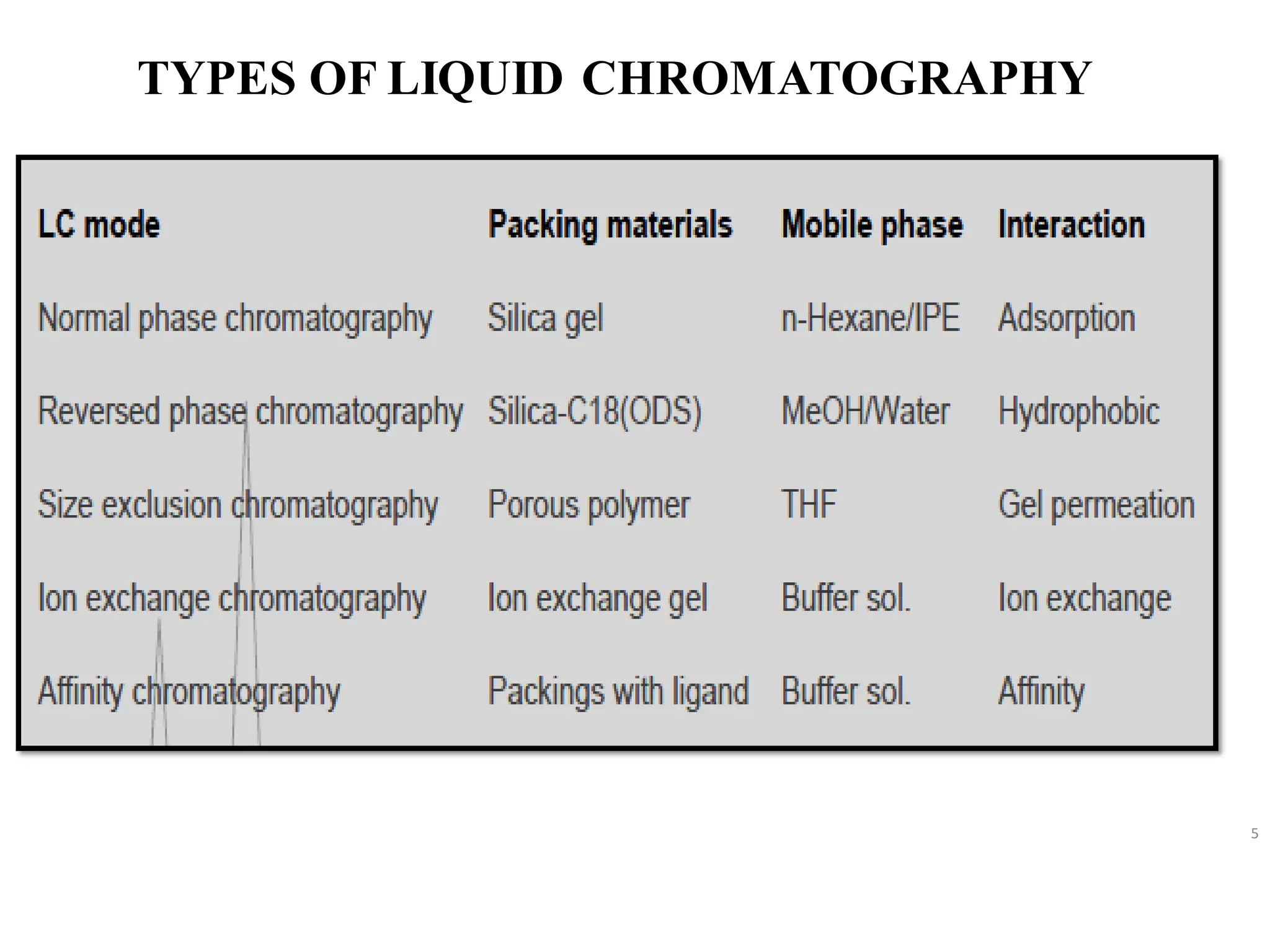
• High Performance Liquid Chromatography [HPLC] is principle is based on adsorption as
well as partition chromatography is depending on the nature of stationary phase, if
stationary phase is solid principle is based on adsorption chromatography and if
stationaryphase is liquidprinciple is based on partitionchromatography.
• It is importantfor determination of volatile and non volatile compounds.
• It is importantfor determination qualitative and quantitative analysis.
• It is important for determination of Retention Time (the time is required , after sample
injectionmaximum angle peak reaches to detector)
8](https://image.slidesharecdn.com/hplc-241225135806-f1bf49c9/75/HIGH-PERFORMANCE-LIQUID-CHROMATOGRAPHY-HPLC-8-2048.jpg)