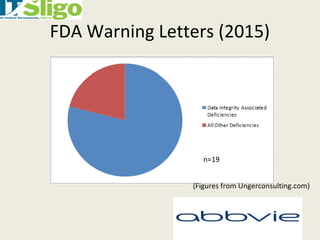
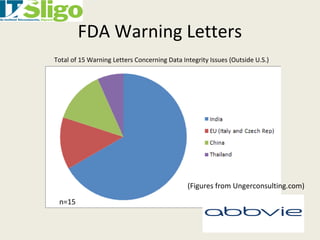
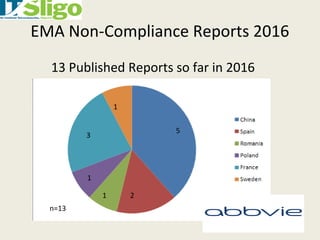
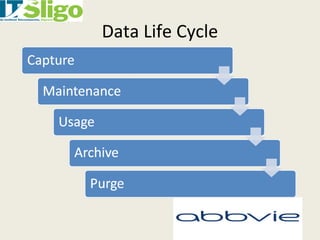
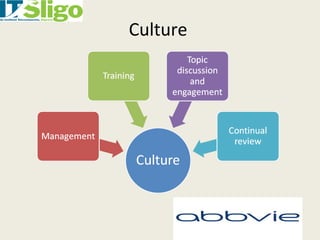
This document discusses data integrity in pharmaceutical quality systems. It defines data and explains its importance in decision making and continual improvement. It notes that the FDA and EMA have issued warning letters and non-compliance reports for data integrity issues. Examples are provided of warning letters issued to two companies for violations like unauthorized data manipulation, lack of audit trails, and insufficient investigations. The causes of data integrity breaches are discussed. It emphasizes establishing a culture of integrity, security protocols, and complying with cGMP guidelines. It provides details on how to properly manage system access, data storage, backups, and recording data according to ALCOA principles. The importance of audit trail software is also covered.