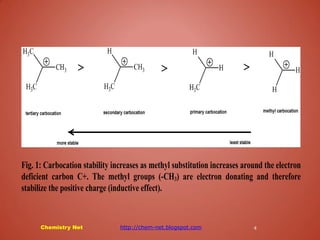
Carbocations are positively charged, electron-deficient species that are highly reactive and unstable. Their stability increases with the number of attached carbon atoms due to inductive effects, resonance from neighboring multiple bonds, and lone pair donation from adjacent atoms. Various stabilization mechanisms include hyperconjugation, charge delocalization, and resonance, which help stabilize these carbocations by allowing charge sharing or electron donation.