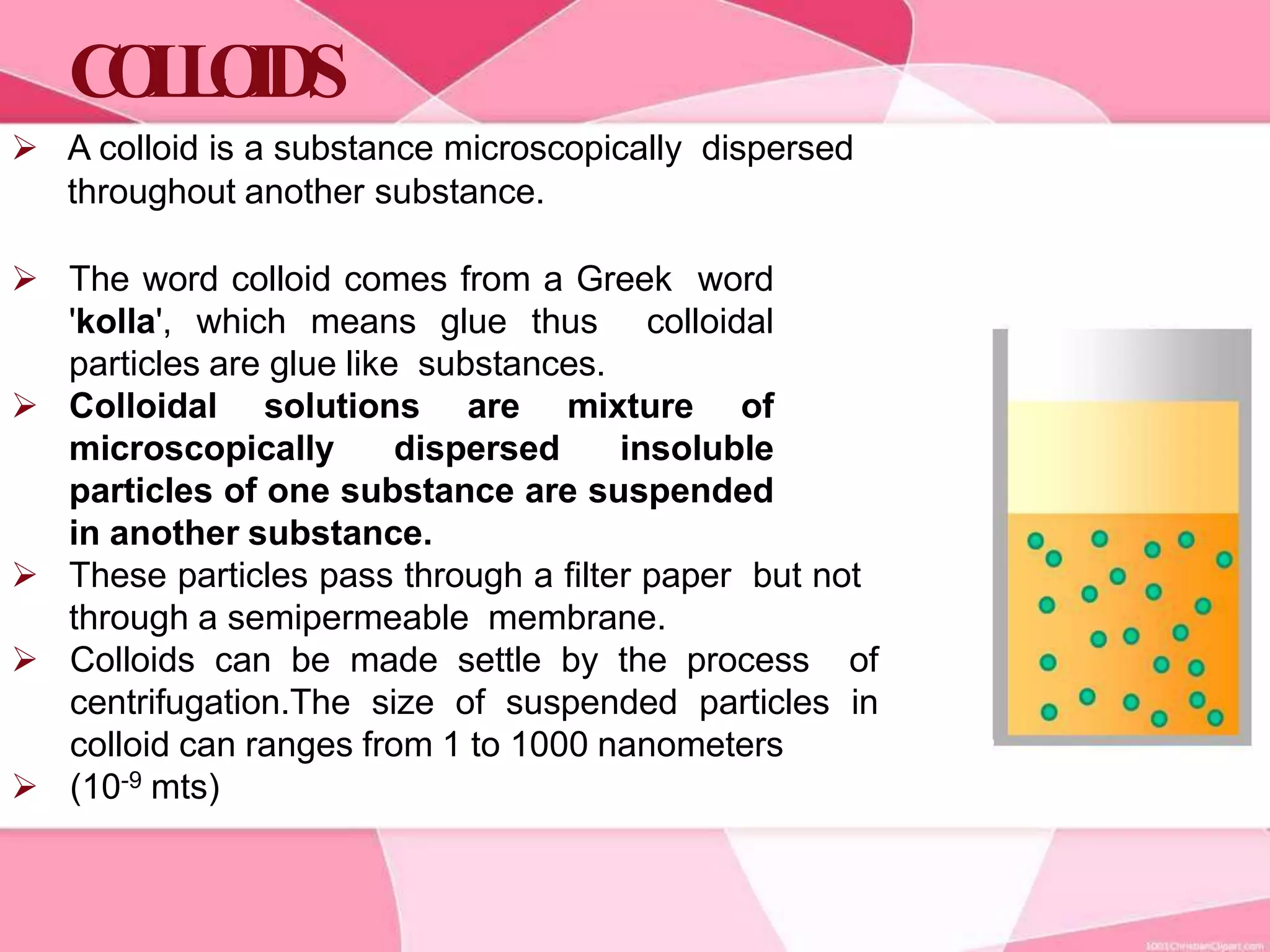
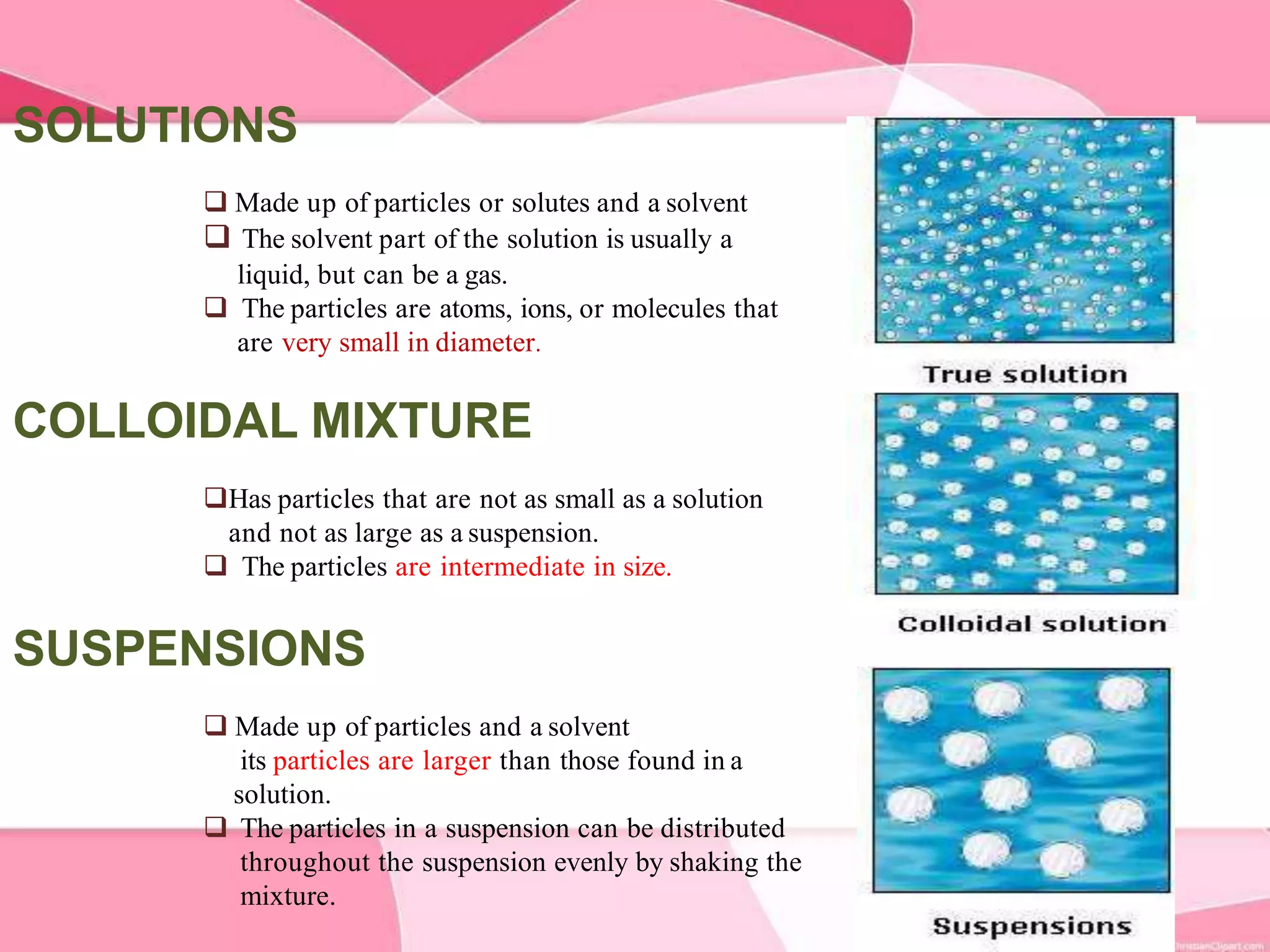
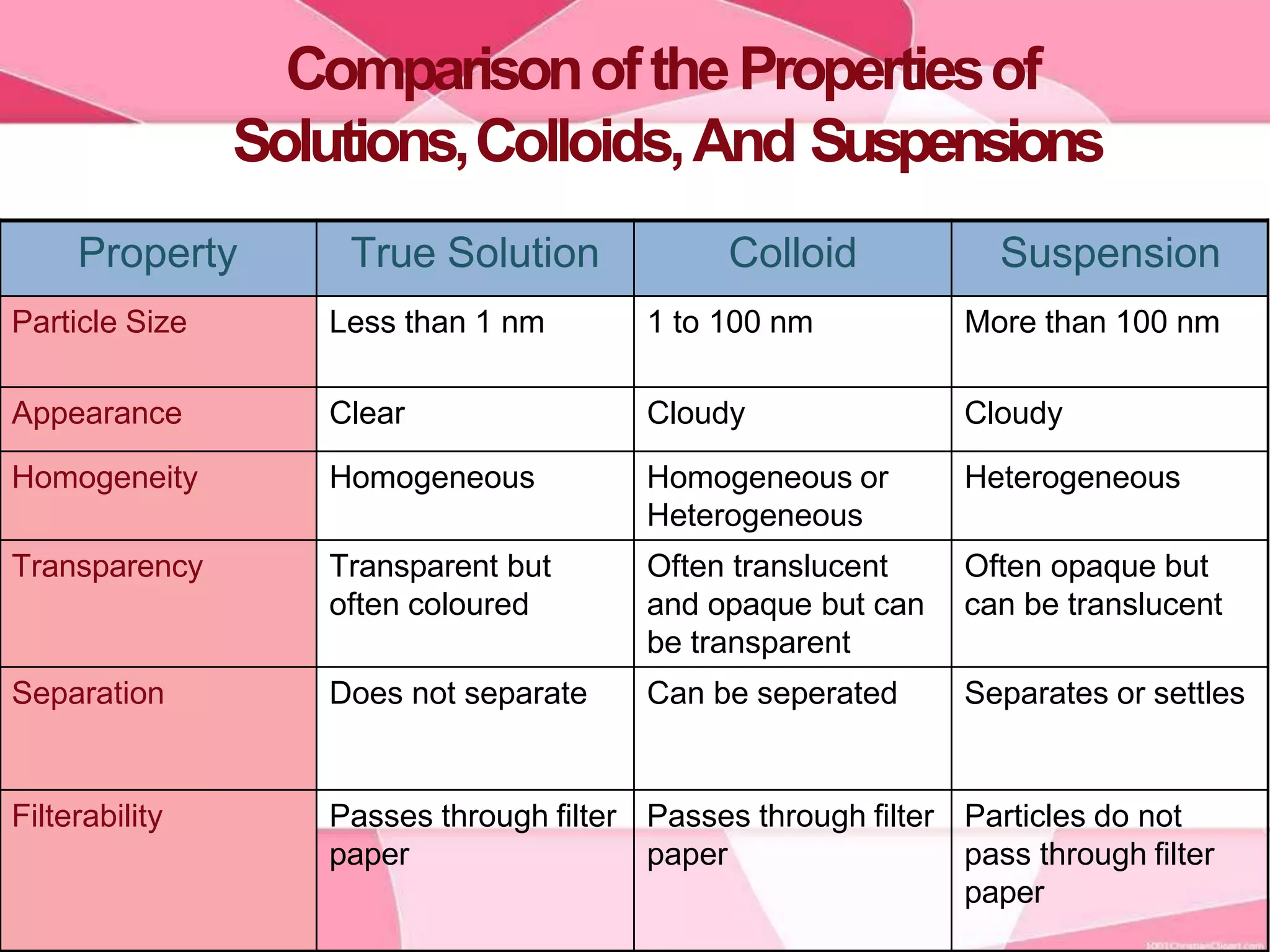
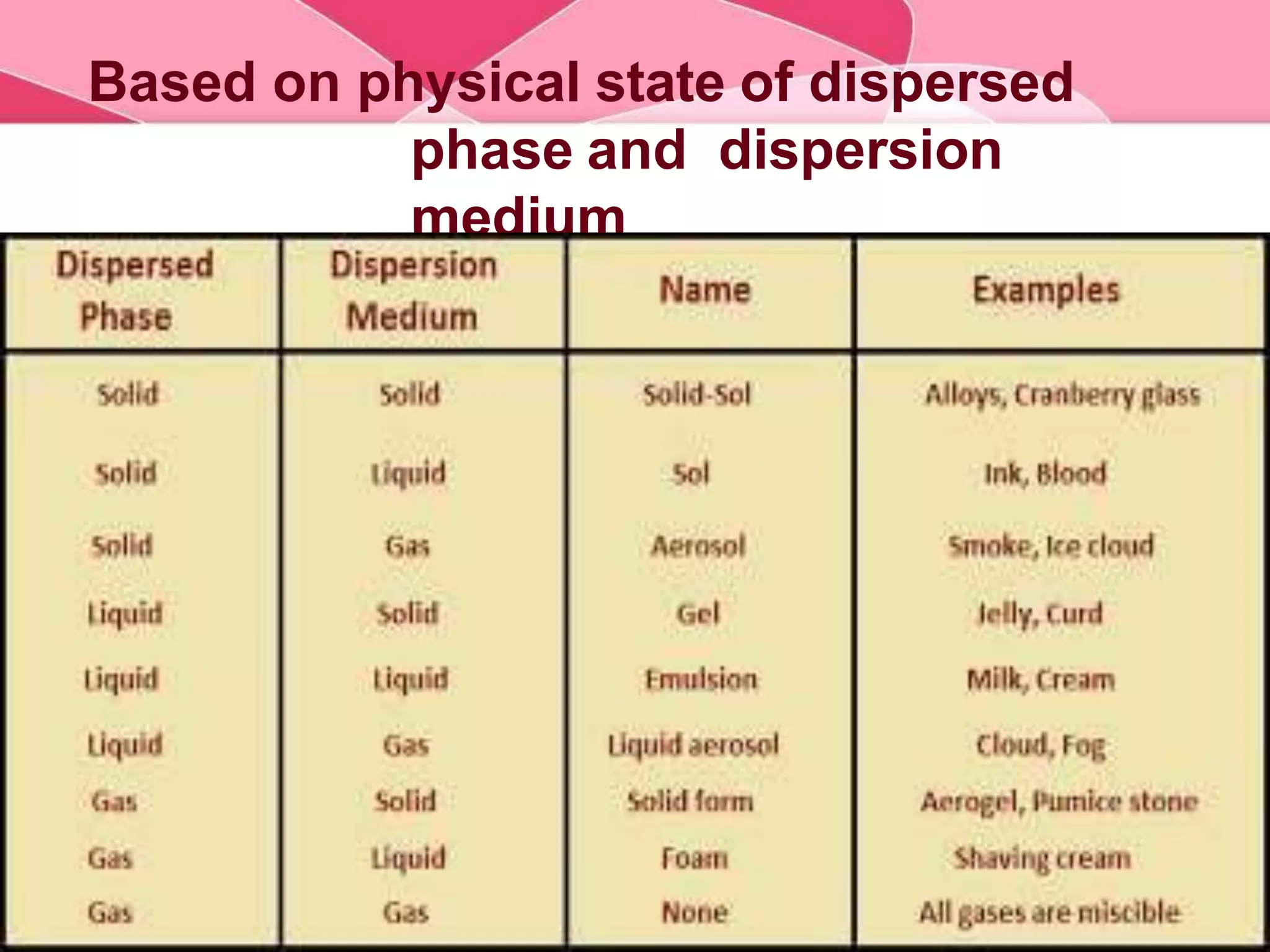
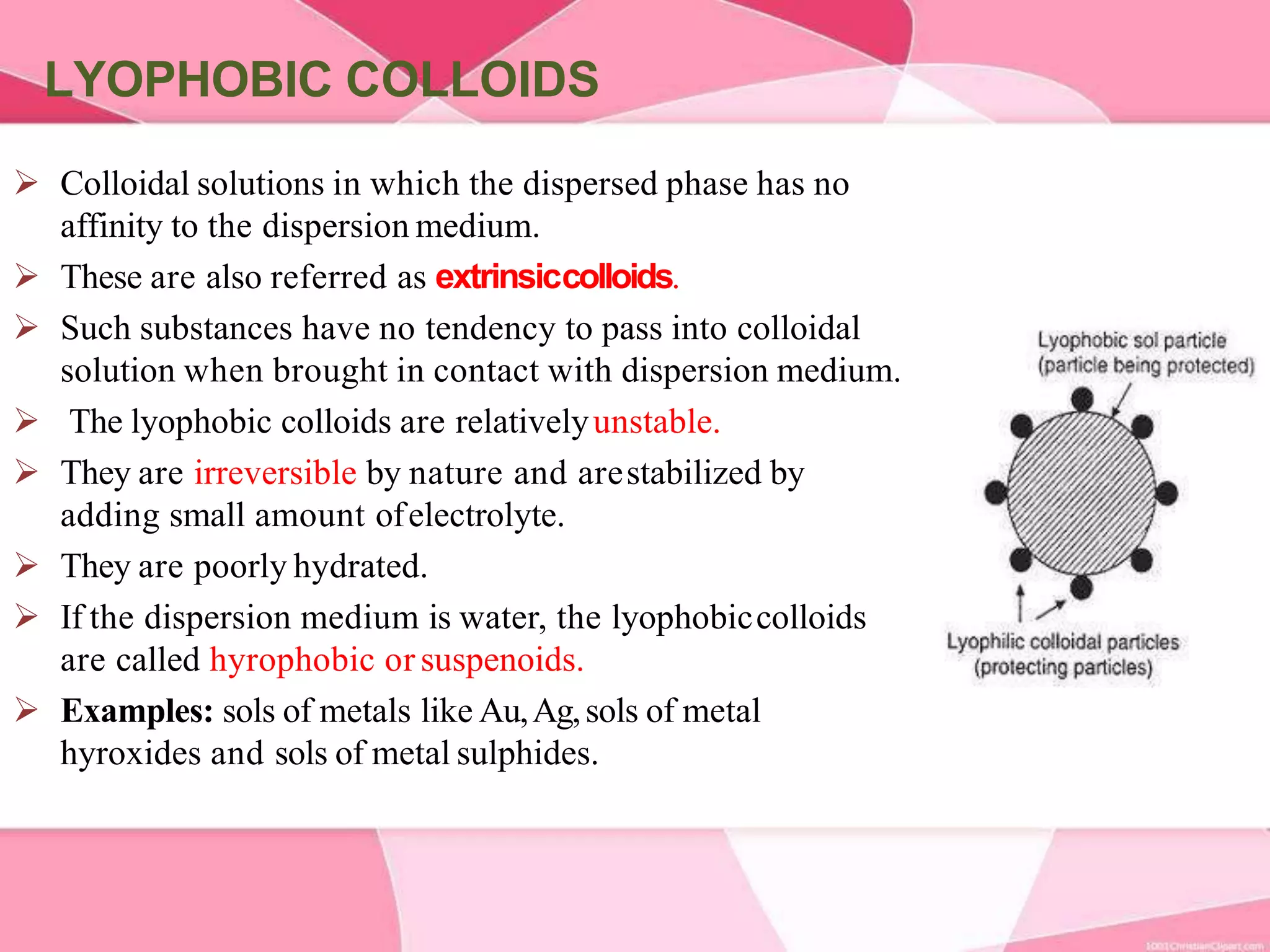
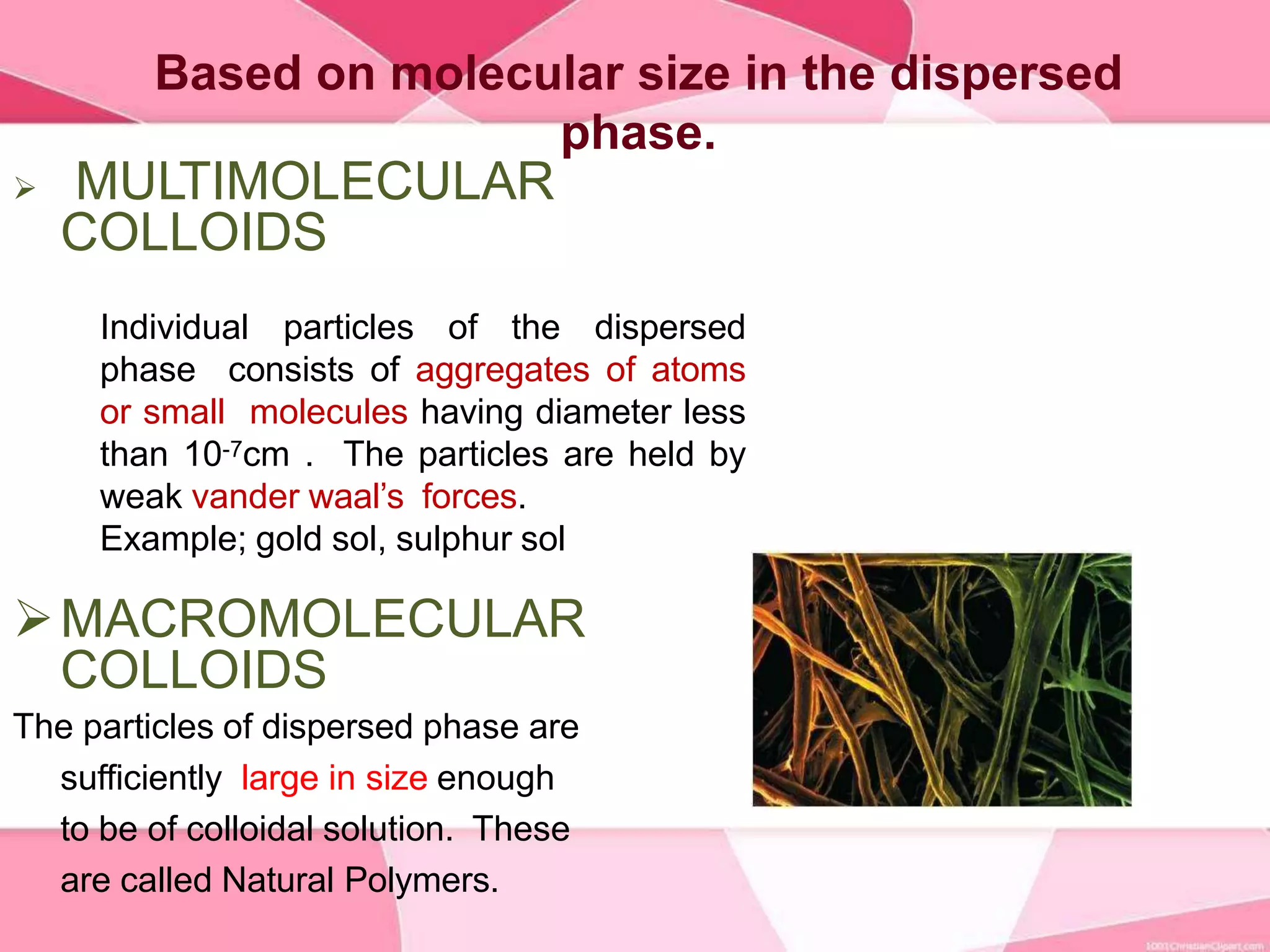
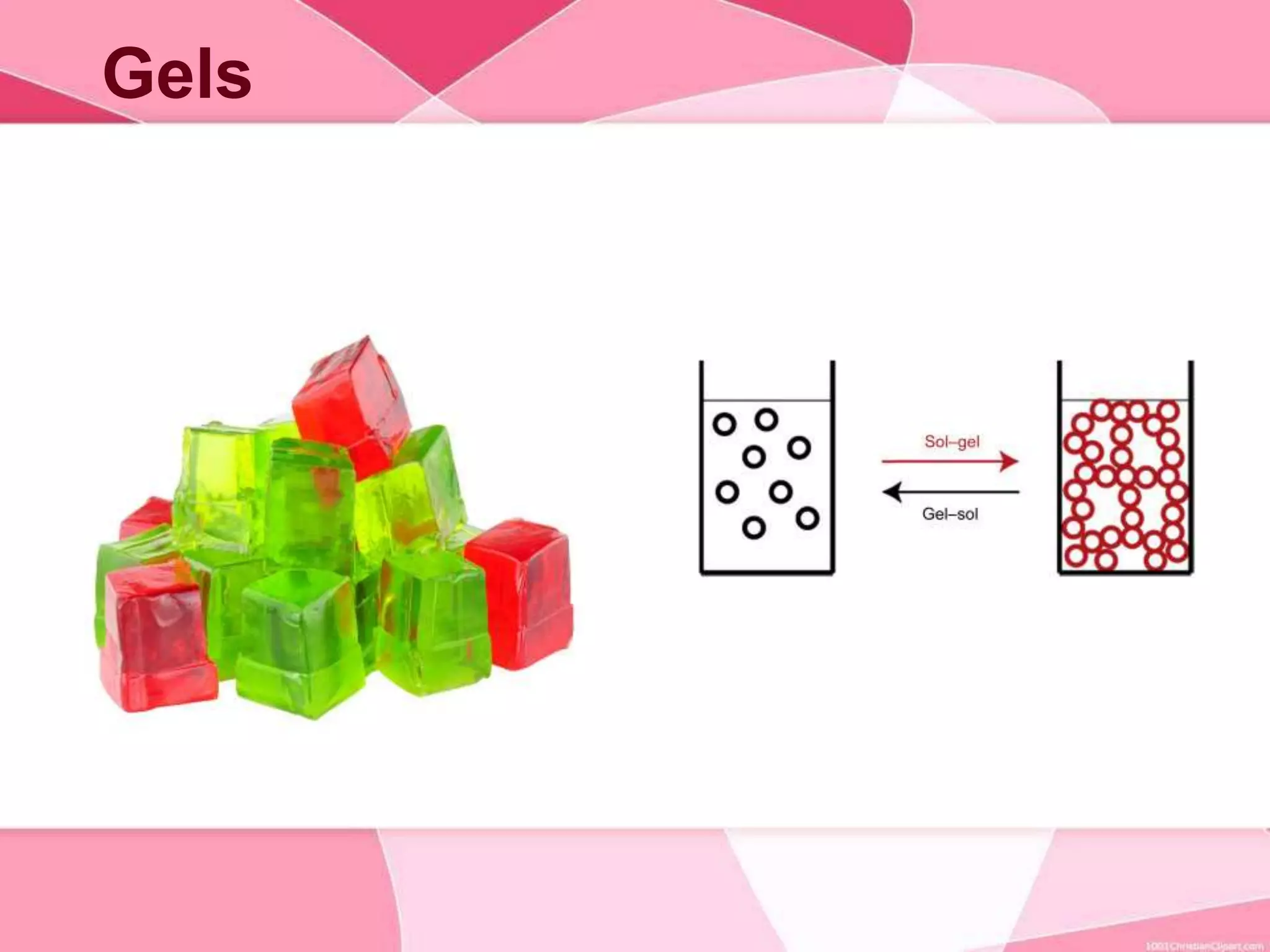
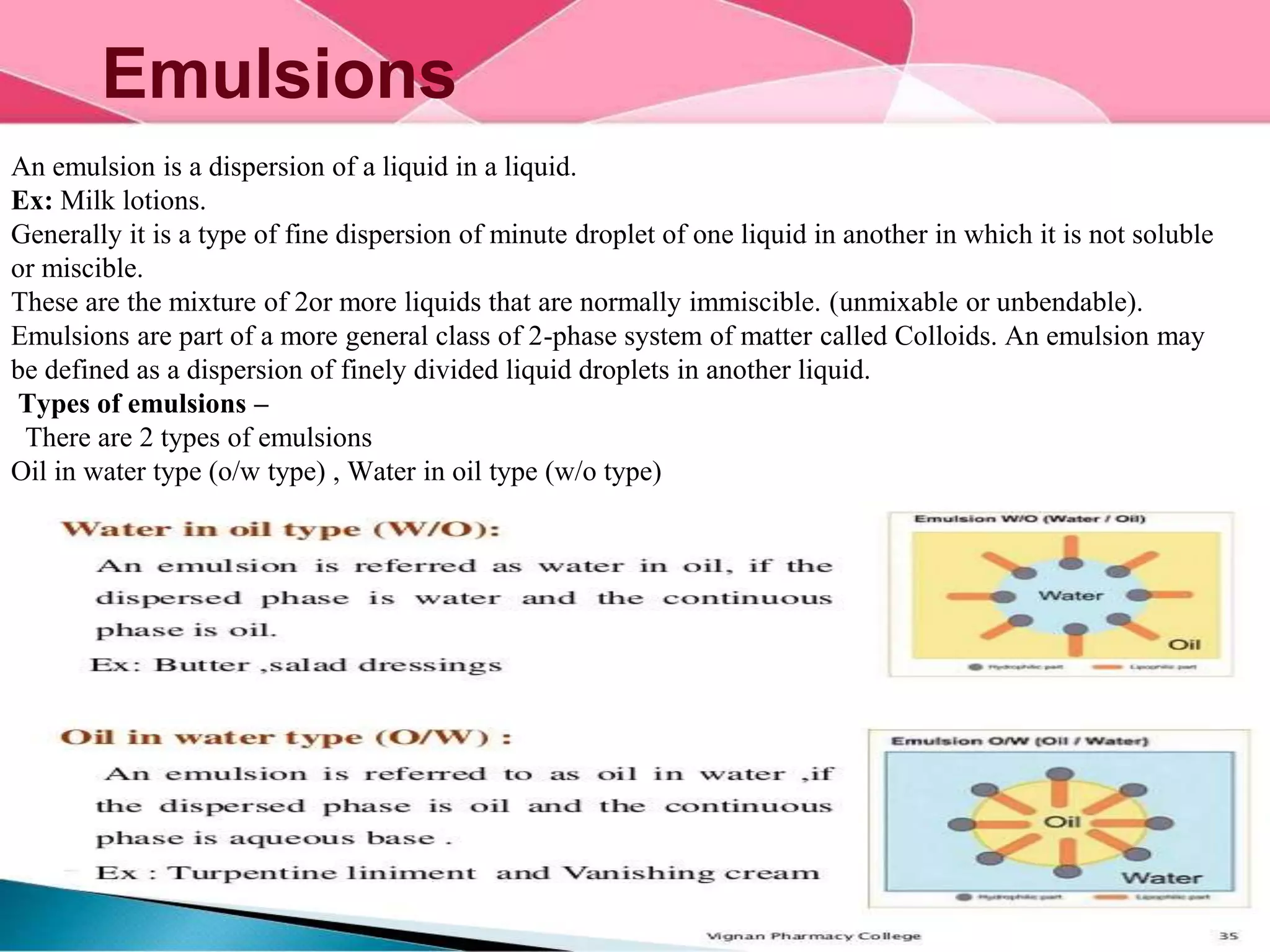
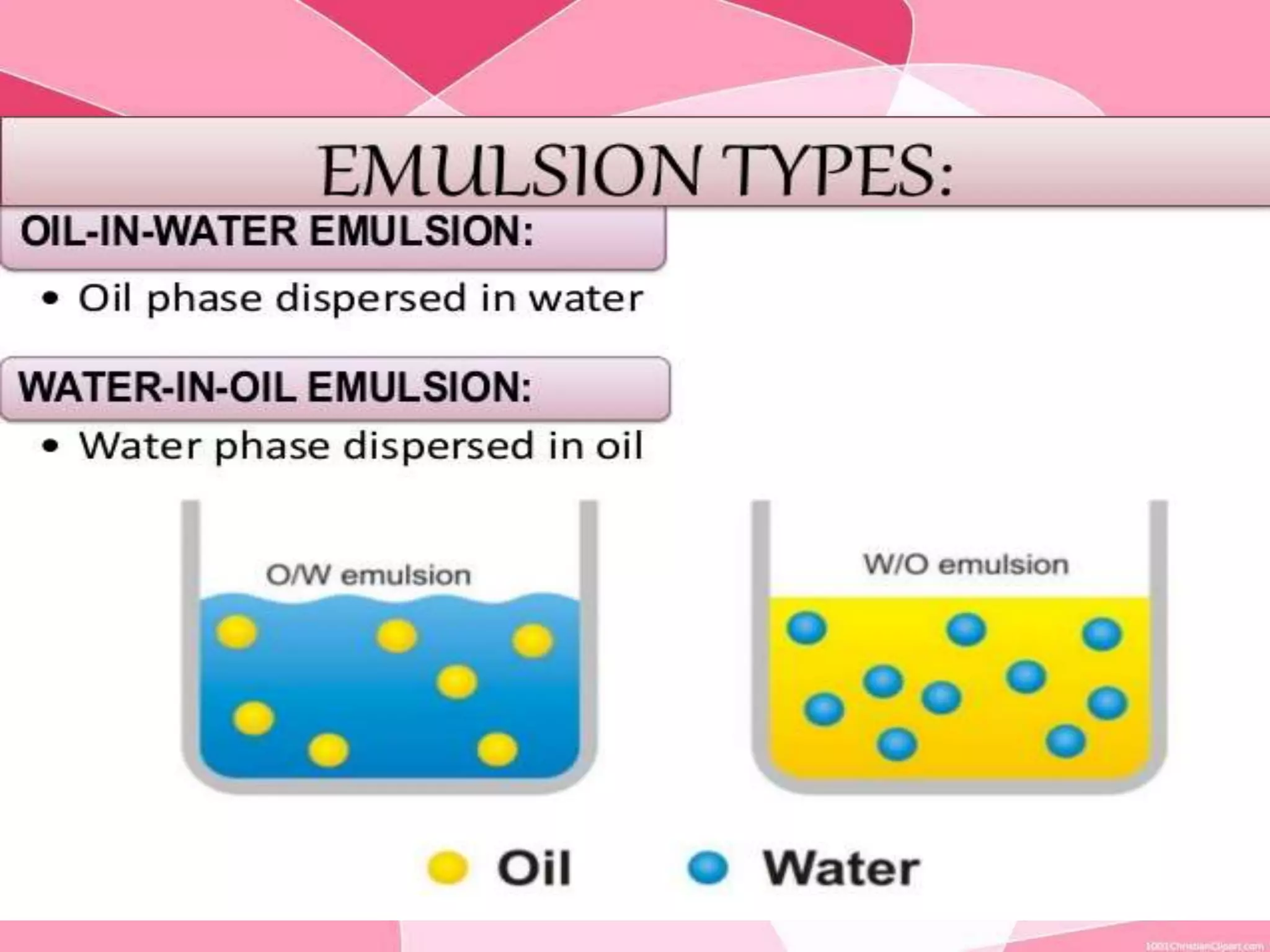
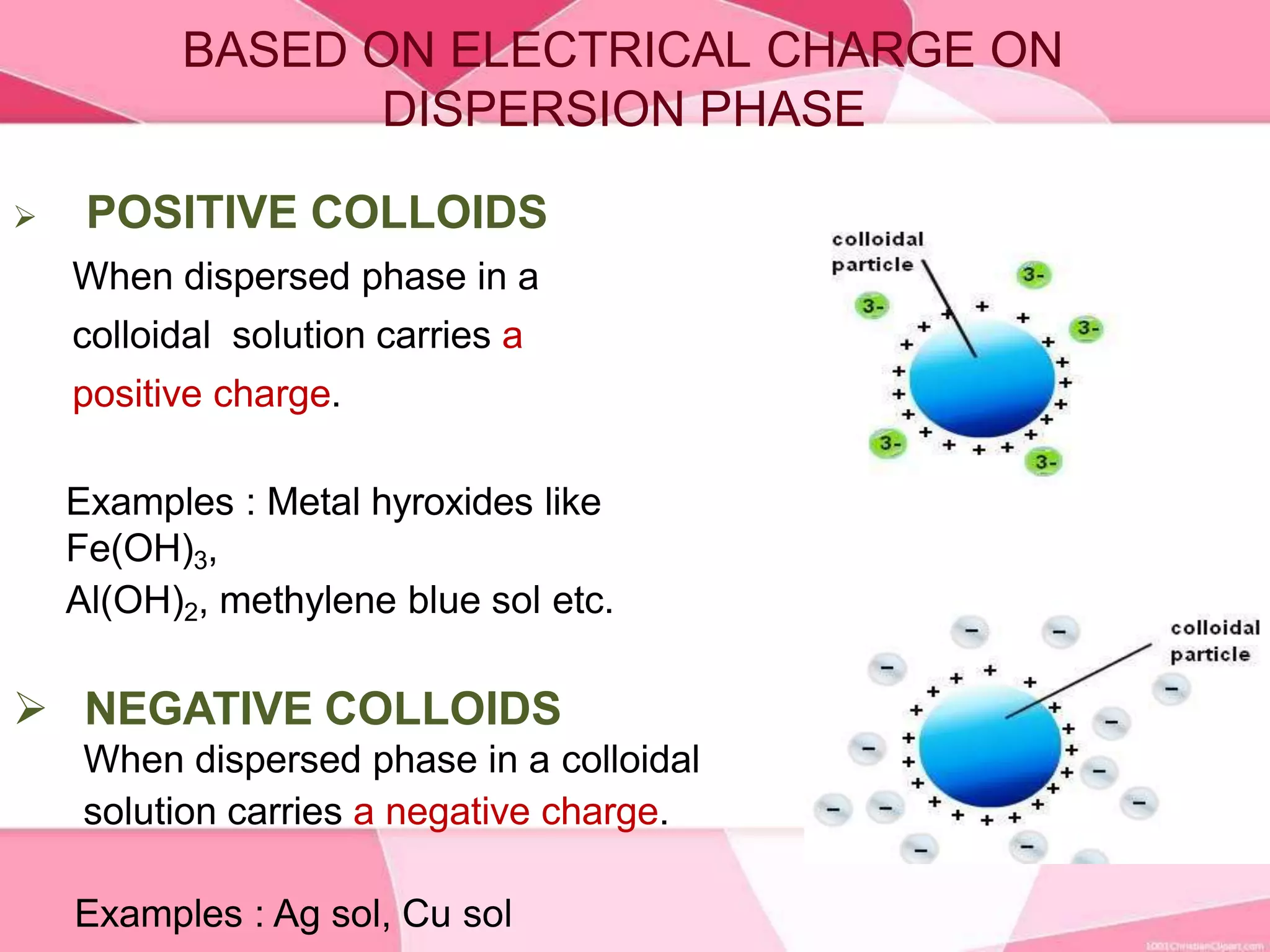
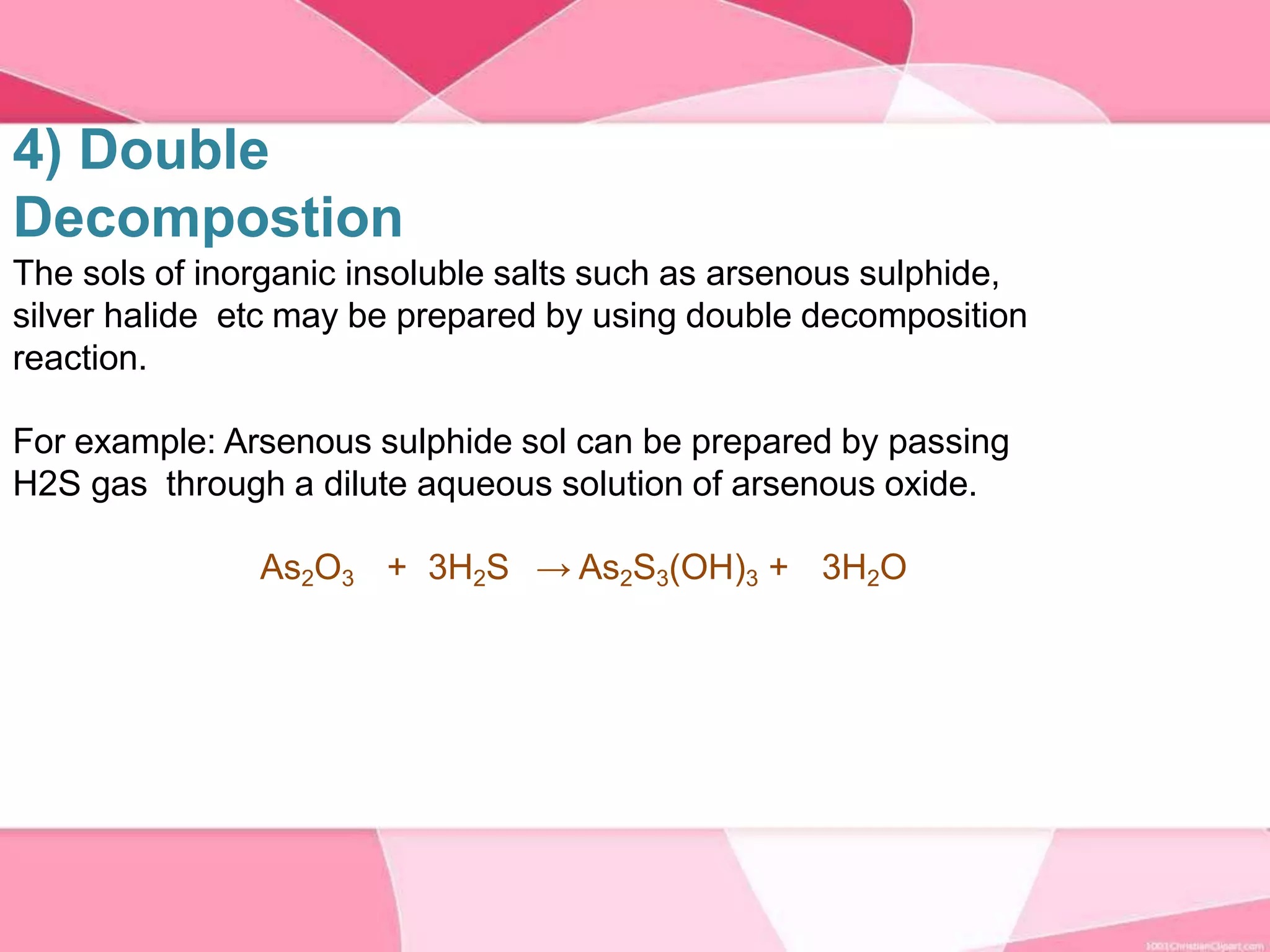
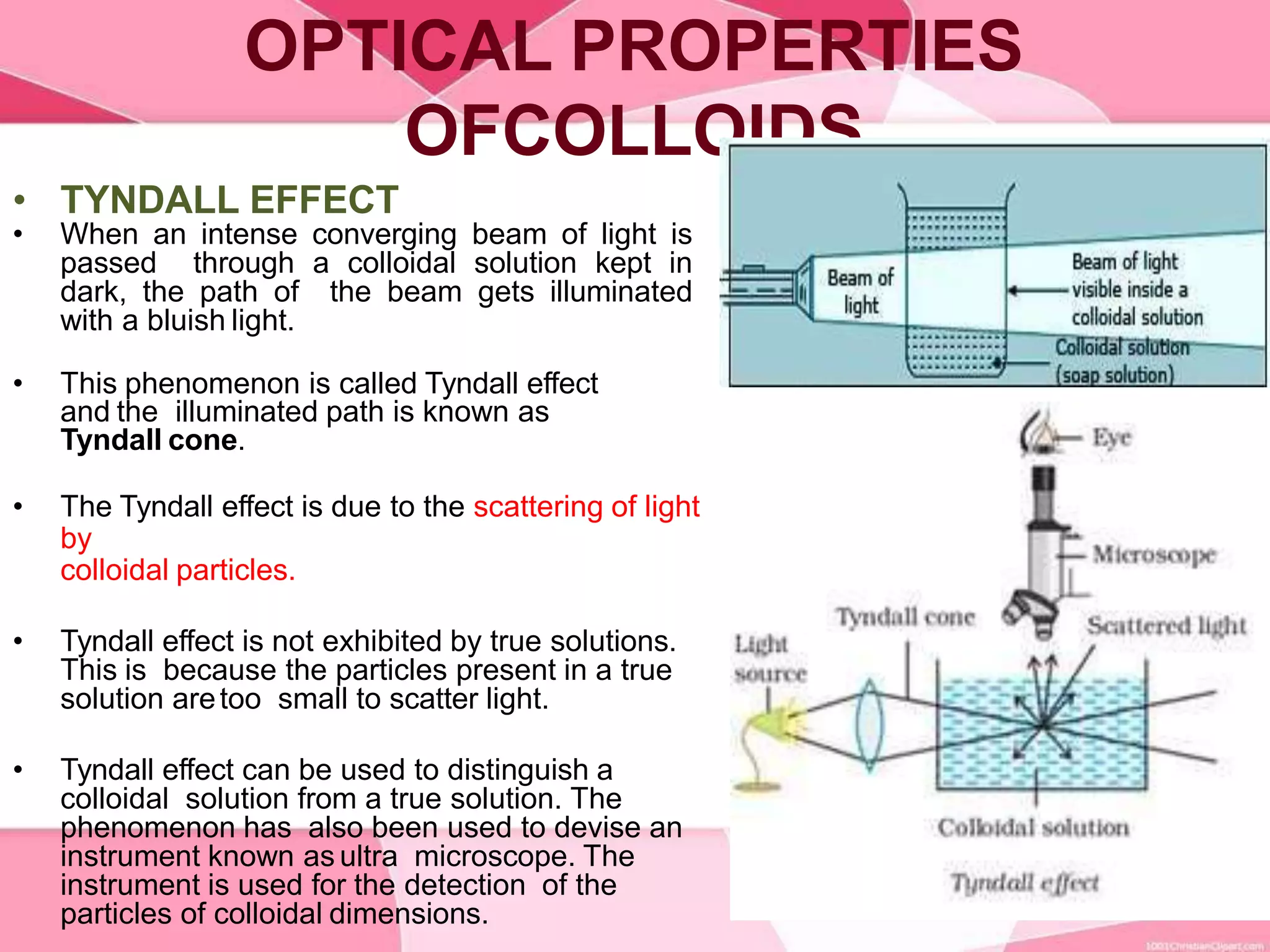
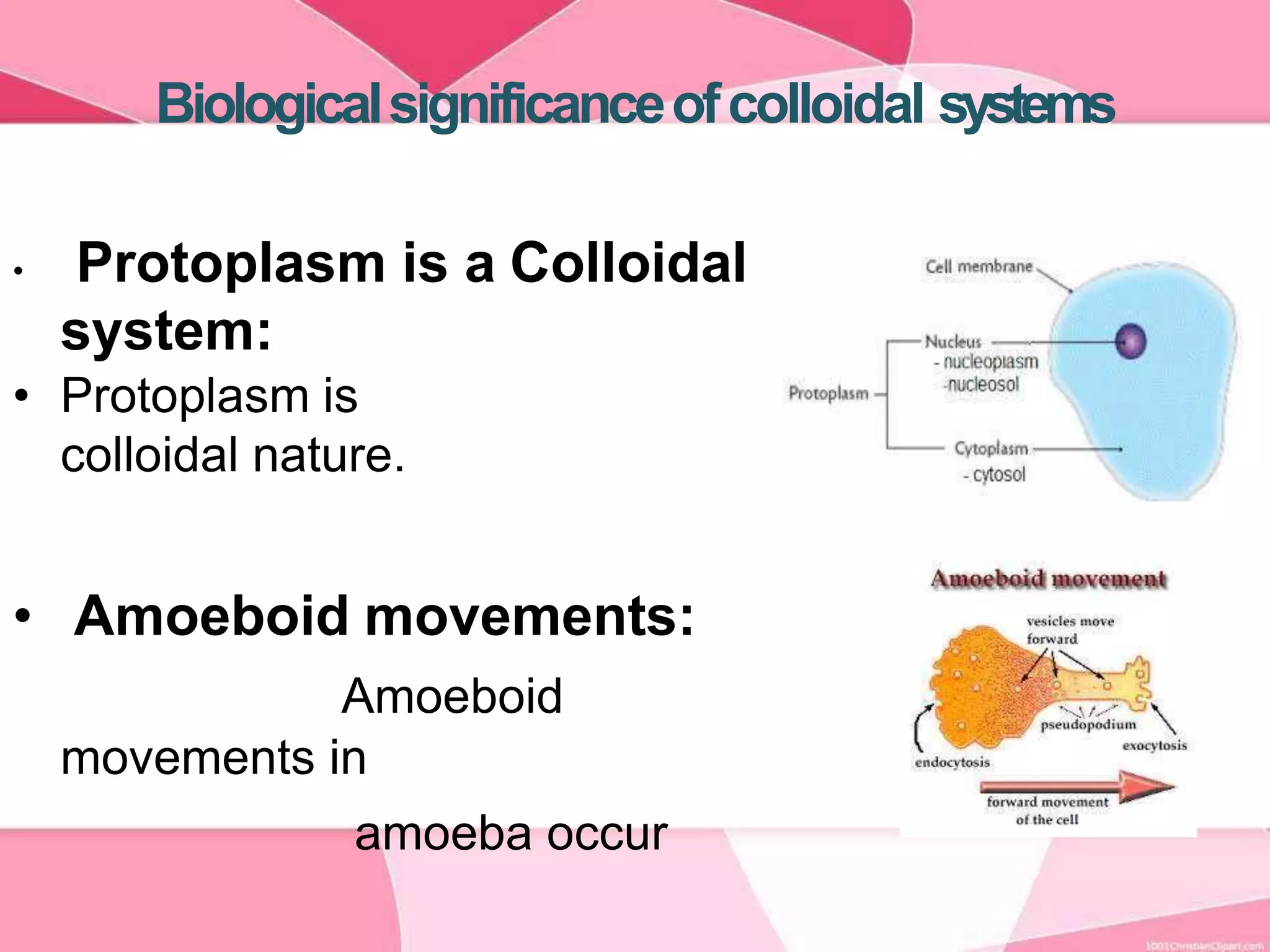
This document provides information about colloids. It defines a colloid as a substance microscopically dispersed throughout another substance. Colloidal solutions contain insoluble particles ranging from 1-1000 nm in size suspended in another substance. Colloids can be classified based on physical state, interaction type, particle size, appearance, or electrical charge. Common examples of colloids include milk, blood, fog and smoke. Colloids can be separated via mechanical dispersion, electrical methods, peptization, or condensation. Properties of colloids depend on whether they are gels, foams, emulsions or aerosols.