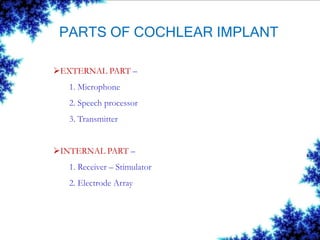
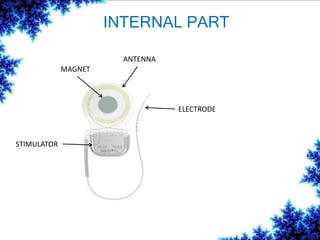
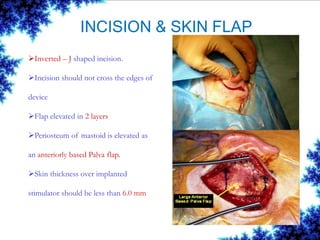
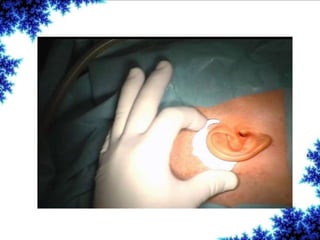
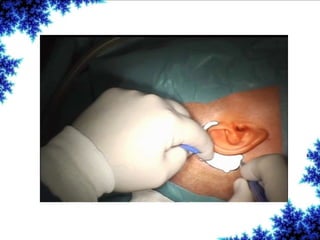
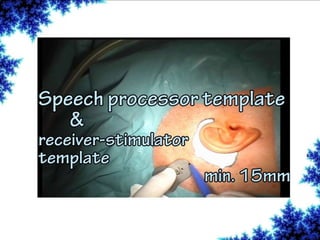
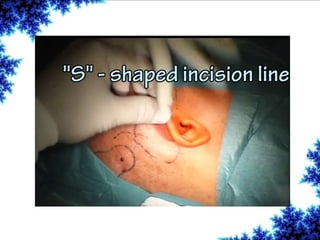
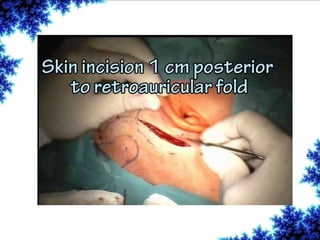
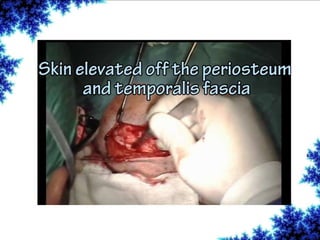
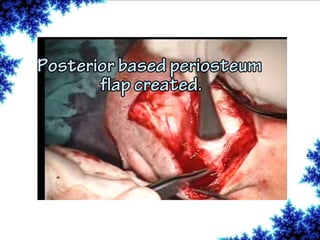
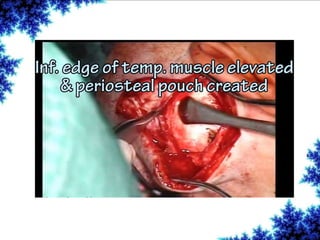
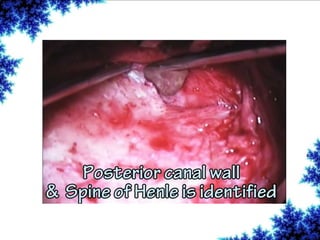
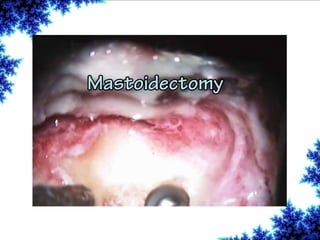
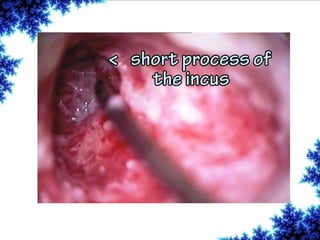
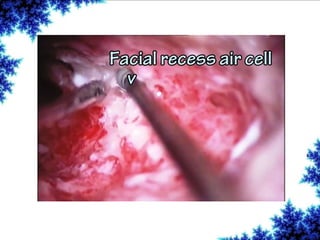
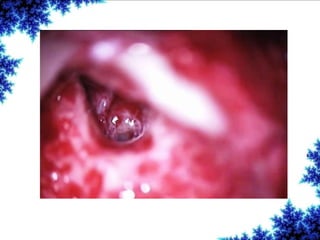
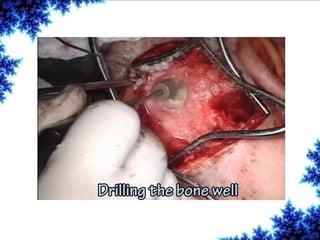
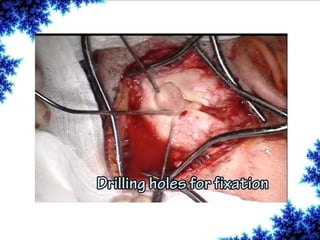
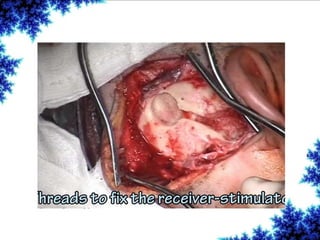
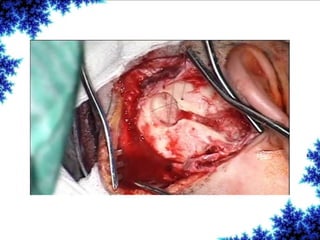
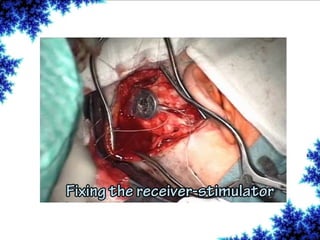
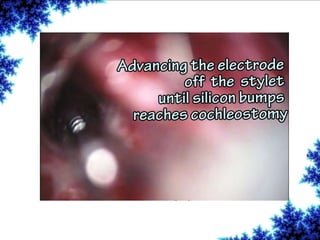
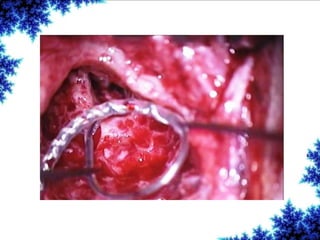
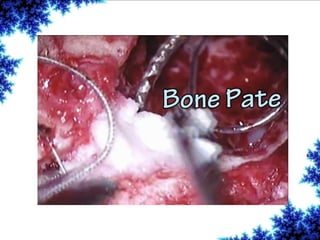
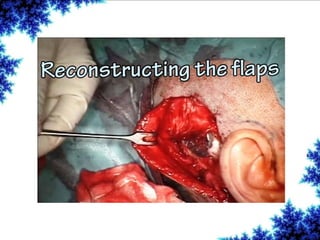
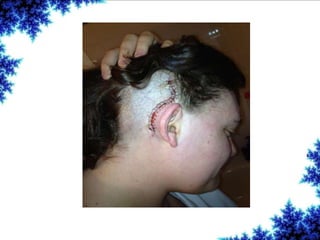
Cochlear implants are bionic devices implanted in the inner ear to bypass damaged auditory cells, providing a sense of sound to individuals with profound hearing loss. The technology has evolved significantly since the first implantation in 1976, with various types available and specific criteria for candidacy in both adults and children. Surgical procedures, postoperative care, and potential complications must be carefully managed to ensure successful outcomes.