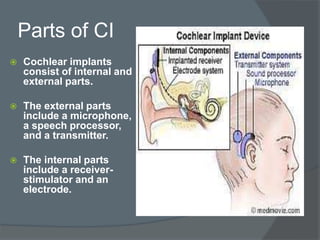
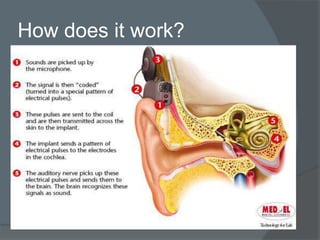
Cochlear implants are electronic devices that bypass damaged parts of the inner ear to provide a sense of sound to someone who is profoundly deaf or severely hard of hearing. They work by directly stimulating the auditory nerve with electrical signals. The first modern cochlear implant was developed in 1978 and provided multiple channels of stimulation, allowing for understanding of speech. Cochlear implants have since improved and are now commonly used worldwide to help many deaf individuals perceive sound and improve their ability to communicate.















![ The first cochlear implant was invented by
Dr. William House, in 1961.[2] In 1964, Blair
Simmons and Robert J. White implanted a
six channel electrode in a patient's cochlea
at Stanford University.[3]
The modern multichannel cochlear implant
was independently developed and
commercialized by Graeme Clark from
Australia and Ingeborg Hochmair and her
future husband, Erwin Hochmair, with the
Hochmairs' first implanted in a person in
December 1977 and Clark's in August 1978](https://image.slidesharecdn.com/cochlearimplant-190221083847/85/Cochlear-implant-17-320.jpg)