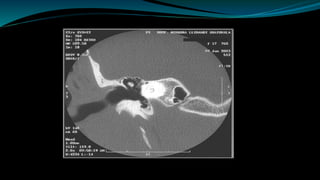
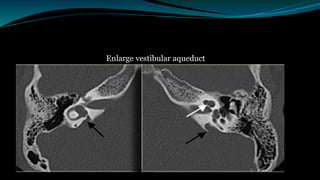
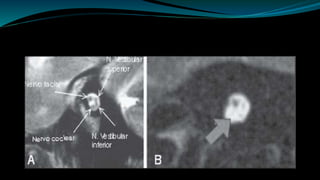
This document provides an overview of cochlear implants, including:
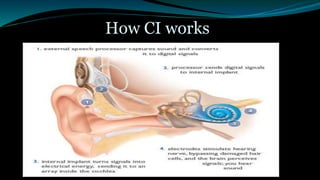
- A cochlear implant is an electronic device that converts sound into electrical signals to stimulate the auditory nerve for people who are profoundly deaf.
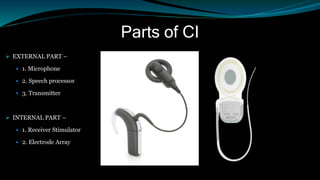
- It has both external and internal components, with the external parts worn behind the ear and the internal parts surgically implanted.
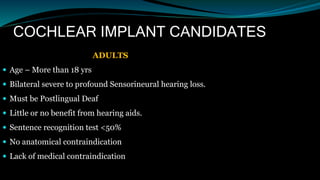
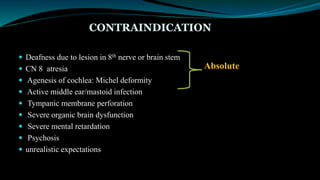
- Candidates for cochlear implants include adults and children over 12 months old with severe-to-profound hearing loss who get limited benefit from hearing aids.
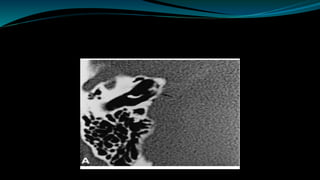
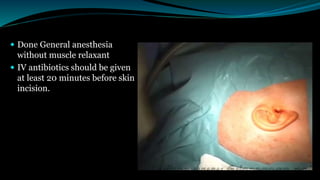
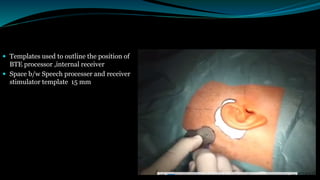
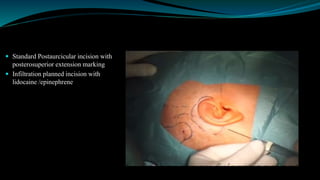
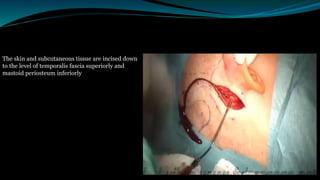
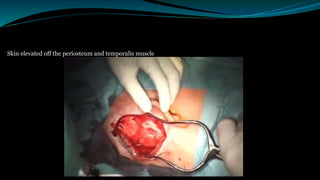
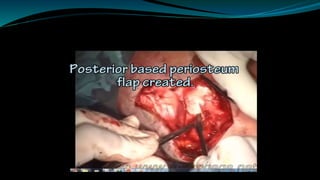
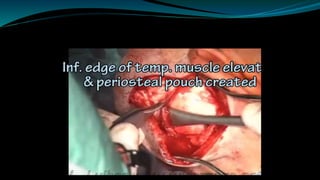
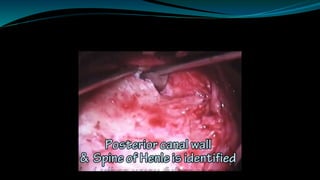
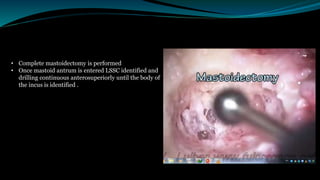
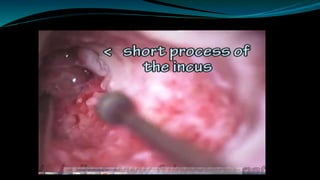
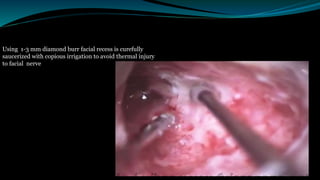
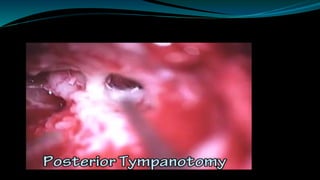
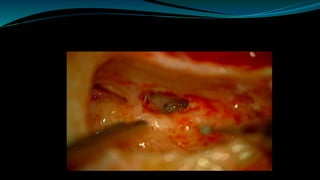
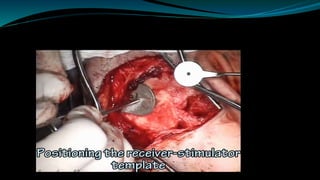
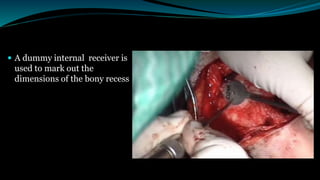
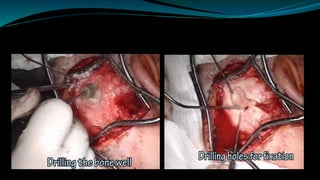
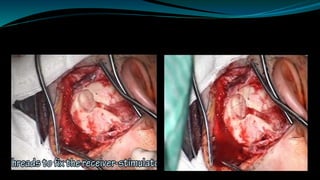
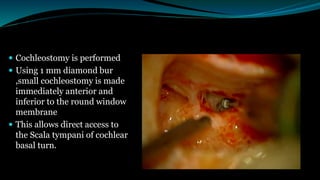
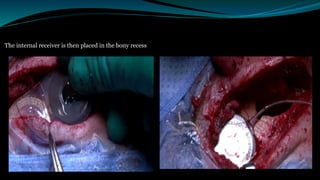
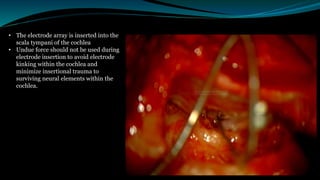
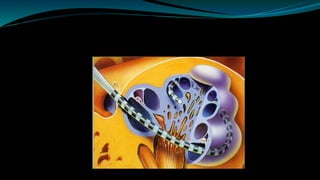
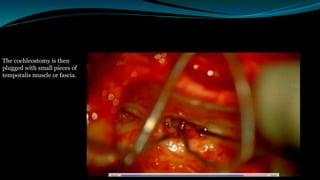
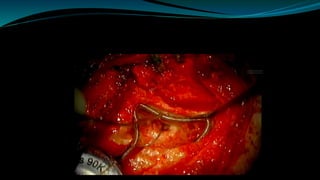
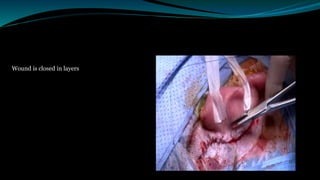
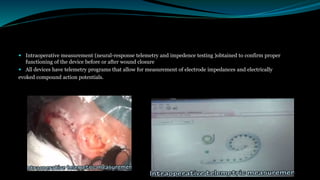
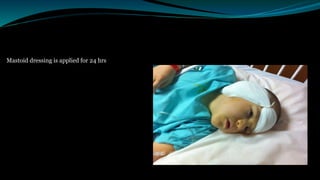
- The surgical procedure to implant the device involves making an incision to access the inner ear and inserting an electrode array to stimulate the auditory nerve. Extensive testing and rehabilitation is required post