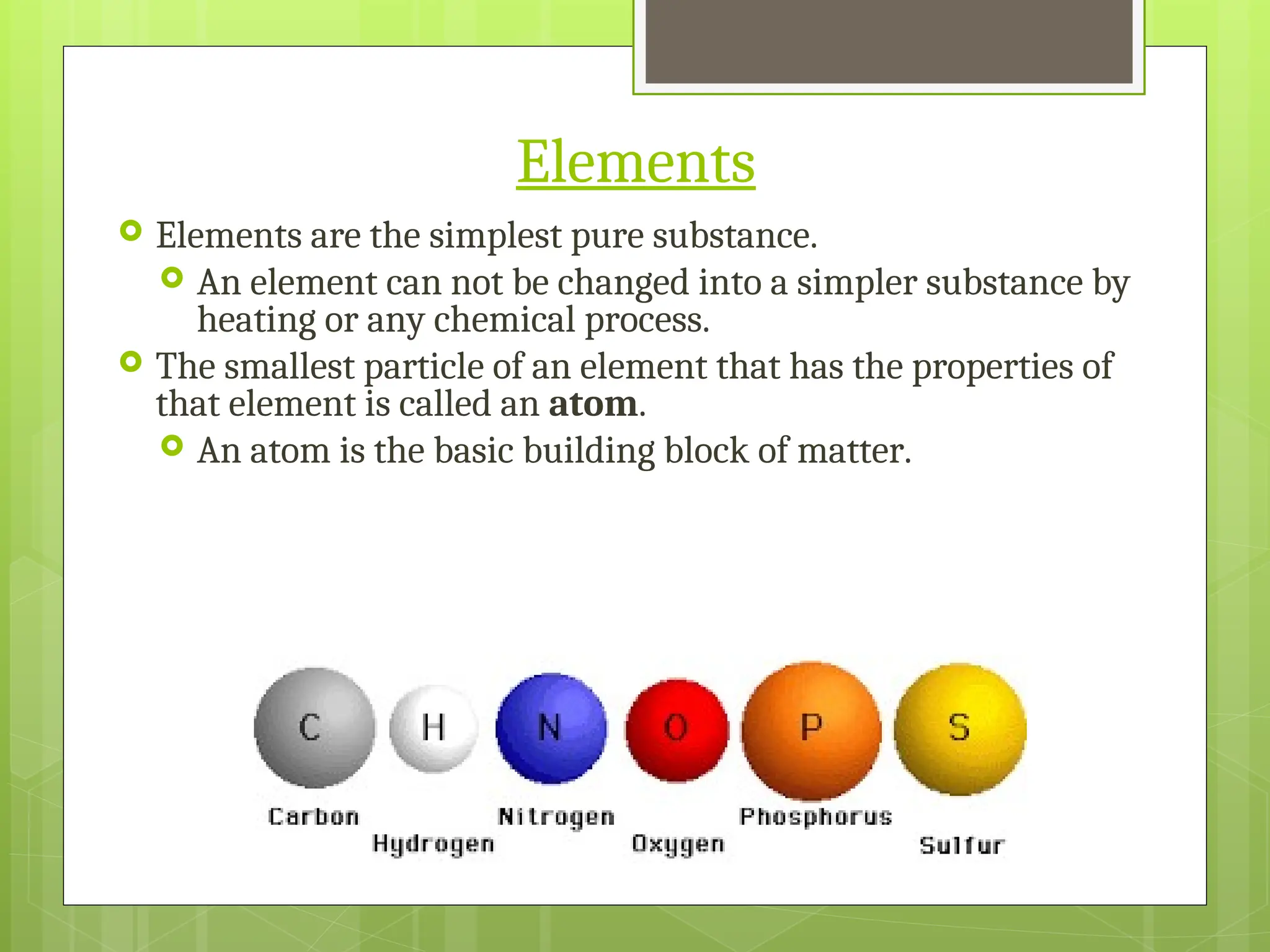
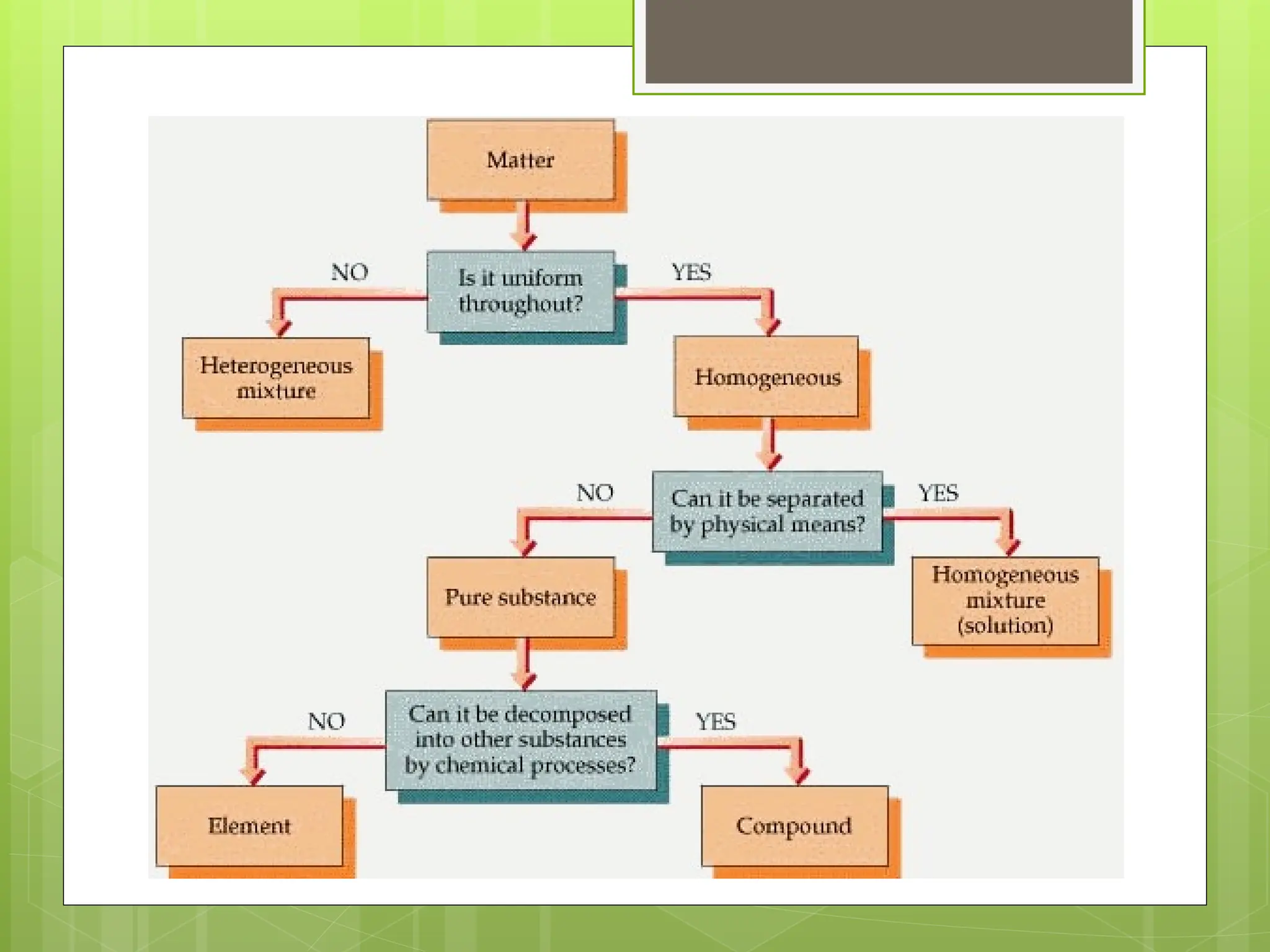
Matter can be classified into mixtures, elements, and compounds based on its composition. Mixtures are combinations of substances that retain their individual properties and can be separated physically, while elements are pure substances that cannot be broken down further. Compounds are pure substances made from two or more elements that can be separated by chemical means.