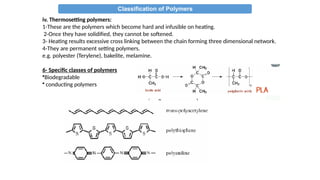
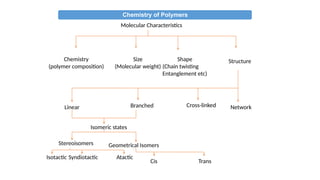
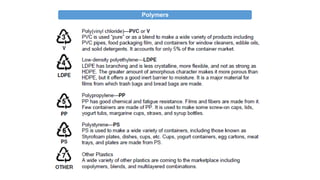
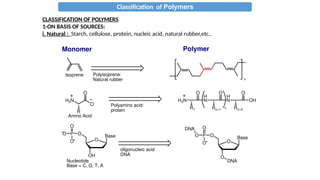
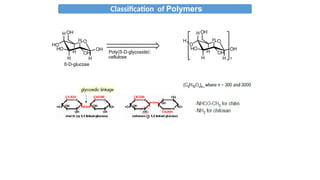
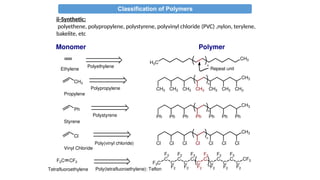
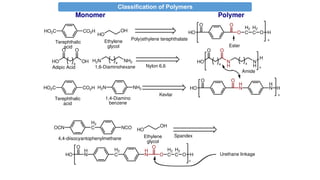
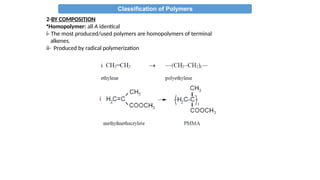
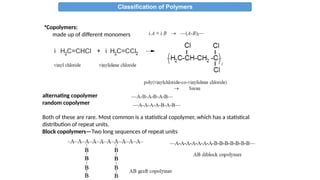
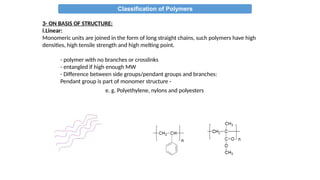
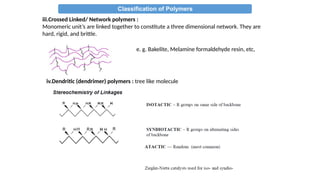
The document provides an overview of polymers, defining them as large molecules formed by the polymerization of monomers and discussing their various classifications based on sources, composition, structure, and polymerization reactions. It highlights the ubiquitous presence and significance of polymers in everyday life, their physical and chemical properties, and their advantages over metals and ceramics. Additionally, it explains the differences between types of polymers such as elastomers, fibers, thermoplastics, and thermosetting polymers.







![Classification of Polymers
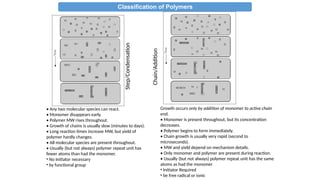
4. BY POLYMERIZATION REACTION: (developed by Carothers)
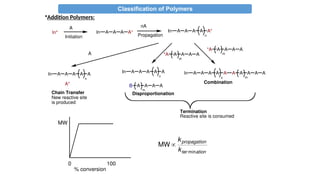
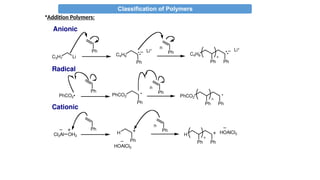
•Addition Polymers:
i-Typically formed by “chain growth” polymerization (sometimes called
addition polymerization)
ii- Same atoms in polymer repeat unit as in monomer
iii-- Usually has an all carbon backbone (w/ pendant groups)
iv-Typically, do not contain functional groups as part of backbone
v- From unsaturated hydrocarbons or olifins
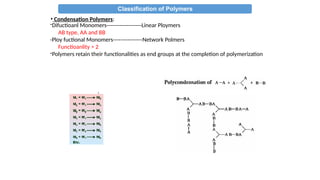
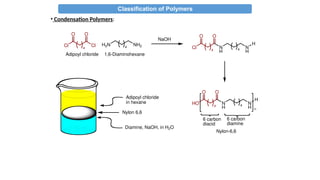
• Condensation Polymers:
i-Typically formed by “step growth” polymerization (sometimes called
condensation polymerization)
ii-Usually fewer atoms vs. the monomer since a small molecule is
eliminated during the polymerization (e.g. water, HCl).
iii-Contain functional groups which react together
e.g. amine, carboxylic acid, hydroxyl, chlorine
iv-Polymer backbone has non-C atoms or NEW
LINKAGES/FUNCTIONAL groups “X”
e.g. amide, ester, ether
A + B X (new functional group formed in bb)
A-R-A + B-R’-B [-R-X-R’-X-]n
A-R-B [-R-X-]n
v- From bi/poly functional monomers](https://image.slidesharecdn.com/chem4134polymerslec1-241211142319-d9442f9e/85/CHEM4134-Polymers-Lec-Condensation-Addition-18-320.jpg)