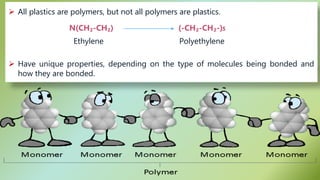
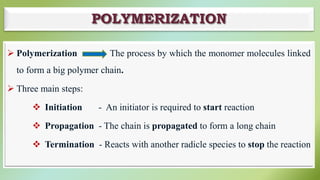
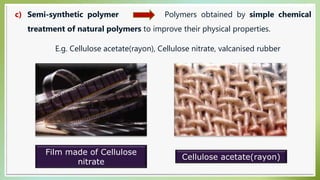
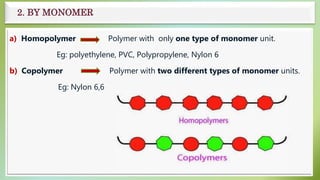
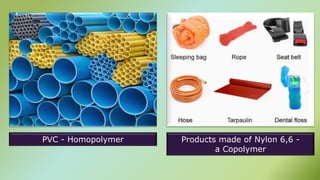
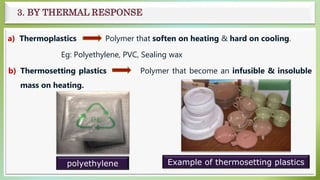
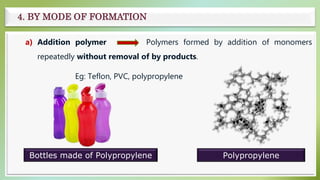
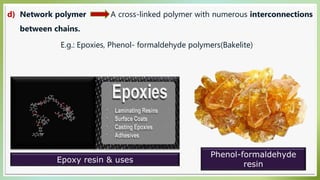
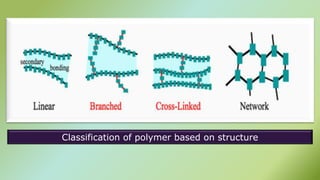
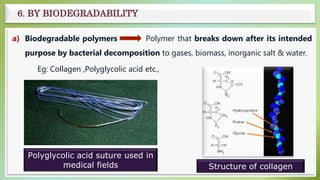
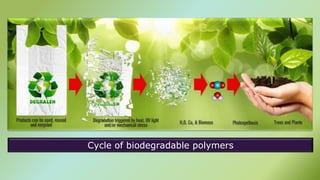
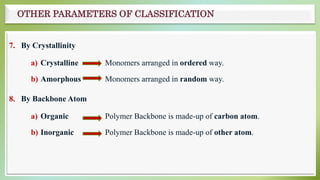
The document presents an overview of polymers, defining them as large molecules composed of repeating subunits called monomers formed through polymerization. It details the classifications of polymers based on origin, monomer type, thermal response, formation mode, structure, and biodegradability. Additionally, it discusses the characteristics of ideal polymers and highlights the importance of properties and modifications for various applications.