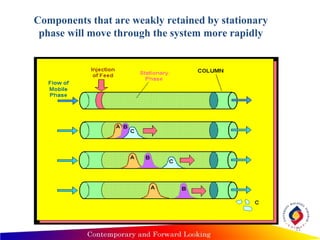
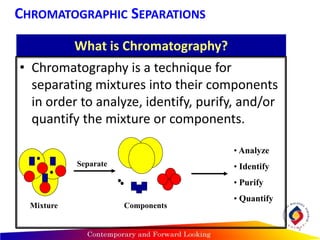
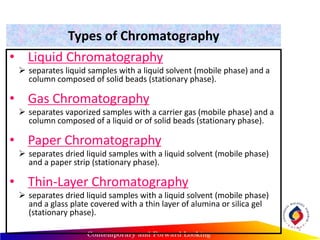
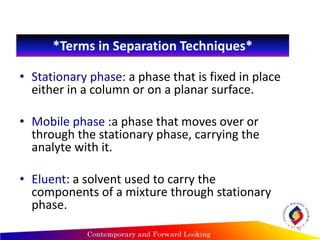
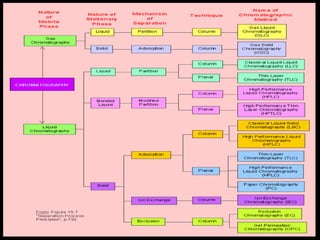
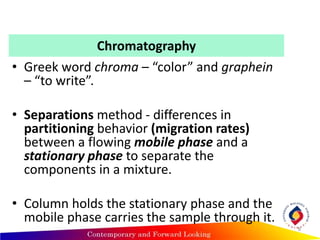
Chromatography is a technique used to separate mixtures into individual components by using differences in how substances partition between a stationary and mobile phase, such as separating compounds by polarity in liquid or gas chromatography, or using paper or thin-layer chromatography. It is used across various scientific fields to analyze, identify, purify, and quantify unknown mixtures and substances. The key concepts are that the stationary phase remains fixed while the mobile phase moves through it, carrying samples by differences in intermolecular interactions based on their polarity.

![Polarity & Intermolecular Attractive
Forces
• More polar compounds will be more attracted to silica gel than non-polar
compounds due to intermolecular attractive forces
- a dipole-dipole interaction.
• The more non-polar compounds will travel more easily and more quickly
through the stationary phase.
• The mobile phase helps carry the compounds through the stationary
phase.
•Separation of compounds in a mixture is possible because compounds
have different polarities. Non-polar compounds will elute first and polar
compounds will elute last.
Si
OH
O
O
O
Si
+
-
-
-
+
Silica gel, [SiO2]n
10](https://image.slidesharecdn.com/chromatography2-150225081031-conversion-gate02/85/Chromatography-techniques-10-320.jpg)