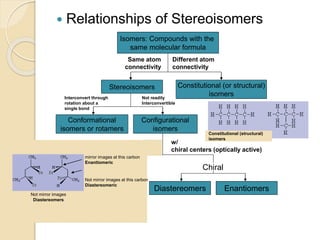
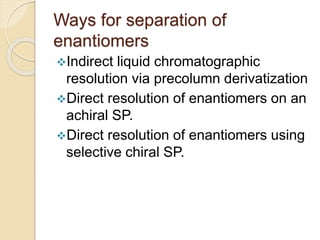
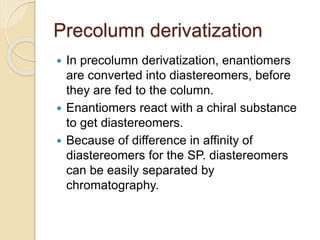
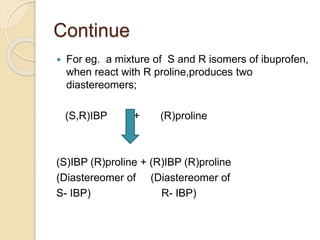
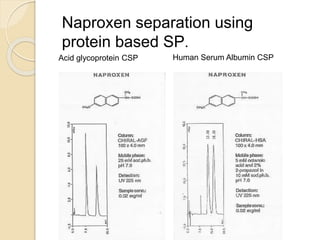
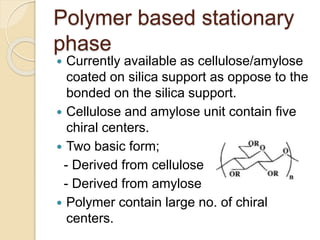
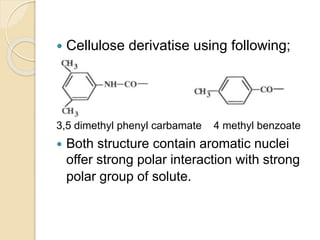
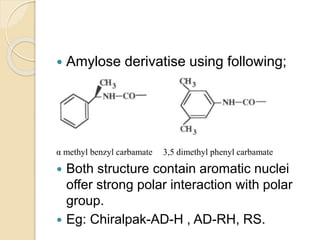
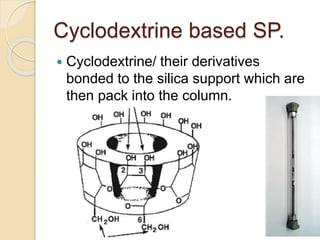
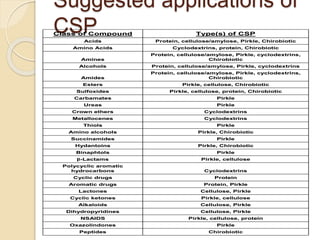
Chiral Chromatography separates enantiomers using chiral stationary phases. It is the most effective method for resolving stereoisomers that have different biological activities. Various types of chiral stationary phases are used, including proteins, cyclodextrins, cellulose, amylose and macrocyclic glycopeptides bonded to silica. These phases interact differently with enantiomers through hydrogen bonding, hydrophobic interactions and other forces. Direct separation on chiral stationary phases or indirect separation after pre-column derivatization can resolve enantiomers that are important to analyze for pharmaceuticals.