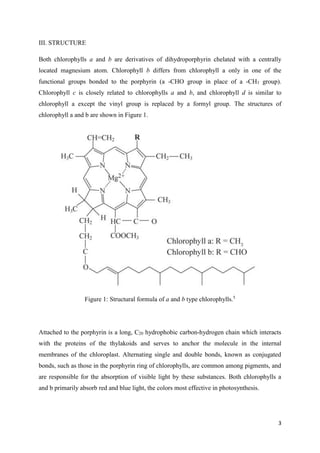
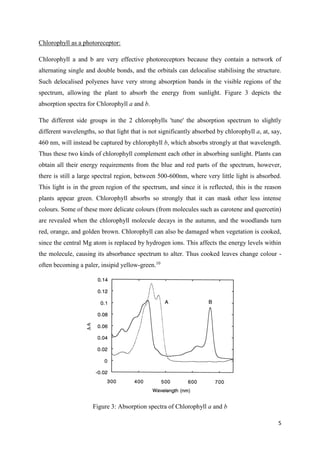
The document discusses chlorophyll, its various types (a, b, c, d, and f), food sources, chemical structure, and its role in photosynthesis. It highlights the effects of processing conditions on chlorophyll stability and degradation, such as heat and acidity, leading to color changes in vegetables. Additionally, it covers methods to preserve and enhance chlorophyll content in food products and the technological advancements in chlorophyll production using algae.




![7
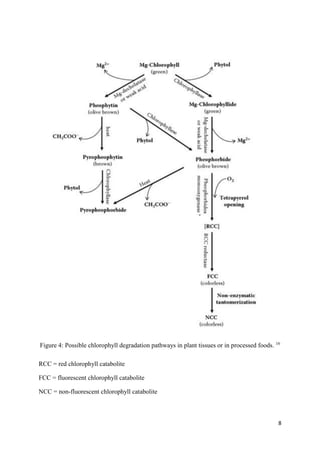
VII. DEGRADATION
Despite the importance of chlorophyll decomposition as a measure of ripening and quality,
the biochemistry of this process remains a biological enigma. Essentially, the process
involves release of chlorophyll from its protein complex followed by dephytlization and
possibly pheophytinization. Oxidation of the ring structure to chlorins occurs and ultimately
colorless end products form. Chlorophylls are degraded in the chloroplast by enzyme-
catalyzed process via pheophorbide a and the red chlorophyll catabolite (RCC) to give
primary fluorescent chlorophyll catabolites (pFCC, or its Cl-epimer, epipFCC). pFCCs are
modified further by unidentified hydroxylating enzymes. When carrying a free propionic acid
group, FCCs are transported into the vacuole where they isomerize by a spontaneous acid
catalyzed reaction to the corresponding nonfluorescent chlorophyll catabolites (NCCs).
EXAMPLES OF CHLOROPHYLL DEGRADARION 15
Fruit maturation (on or off the plant):
Ripening of tomatoes (chlorophyll colorless compounds); induced by ethylene
De-greening of bananas (chlorophyll colorless compounds); induced by ethylene
De-greening of lemons and oranges (chlorophyll colorless compounds); induced
by ethylene
Processing:
Canning of peas [chlorophyll pheophytin (olive brown)]; induced by heat
Brining of olives [chlorophyll pheophytin (olive brown) pheophorbide (olive
brown) and chlorophyll chlorophyllide (blue green) pheophorbide]; induced by
acid and chlorophyllase
Blanching of snap beans, turnip greens and okra [chlorophyll pheophytin (olive
brown)]; induced by heat
Coleslaw processing [chlorophyll pheophytin (olive brown) pheophorbide
(olive brown)]; induced by acid and chlorophyllase
Senescence:
Ready-to-eat salads (chlorophyll colorless compounds); induced by ethylene
Stored cabbage heads (chlorophyll colorless compounds); induced by ethylene](https://image.slidesharecdn.com/chlorophyll-180219173442/85/Chlorophyll-7-320.jpg)