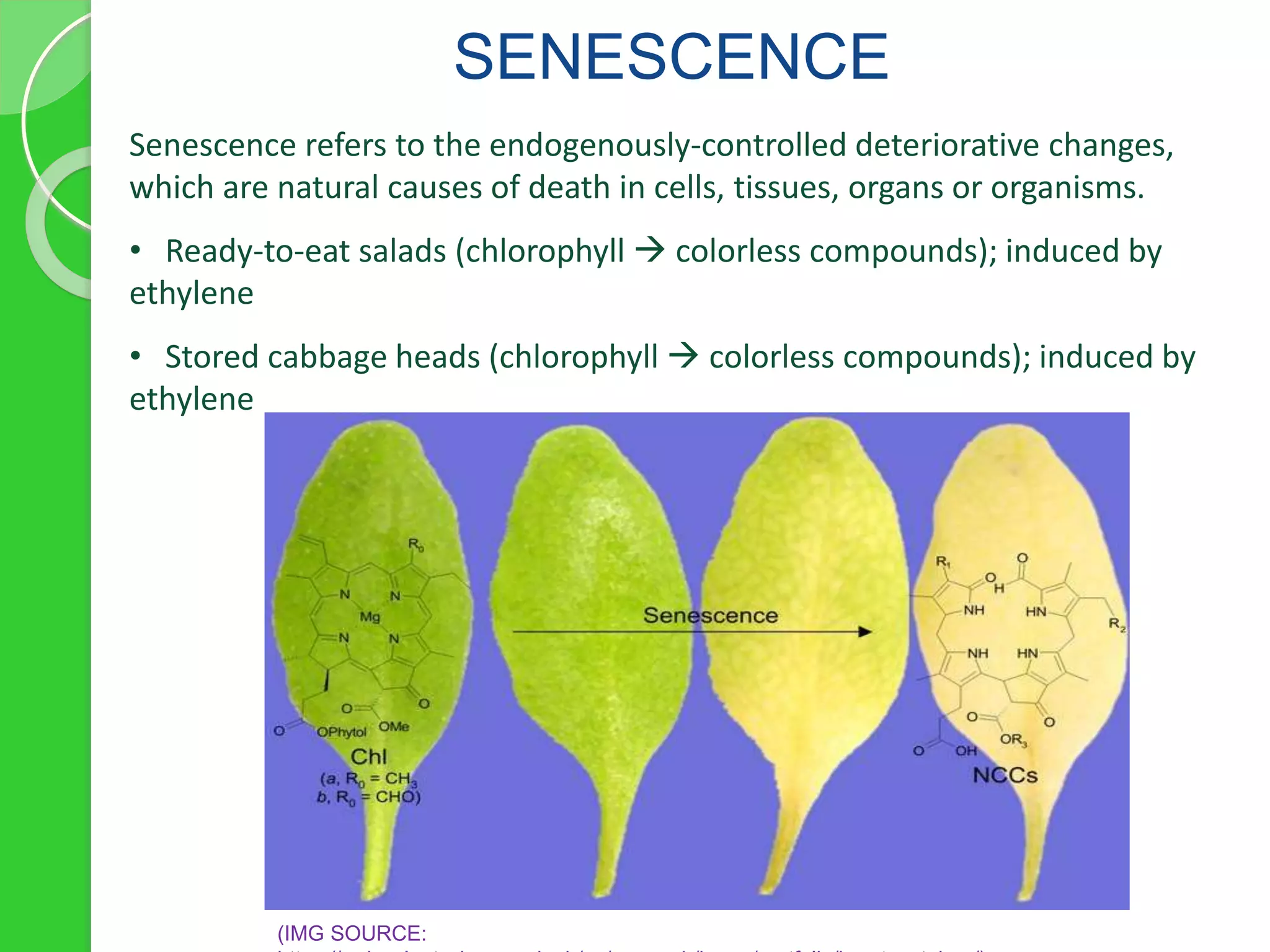
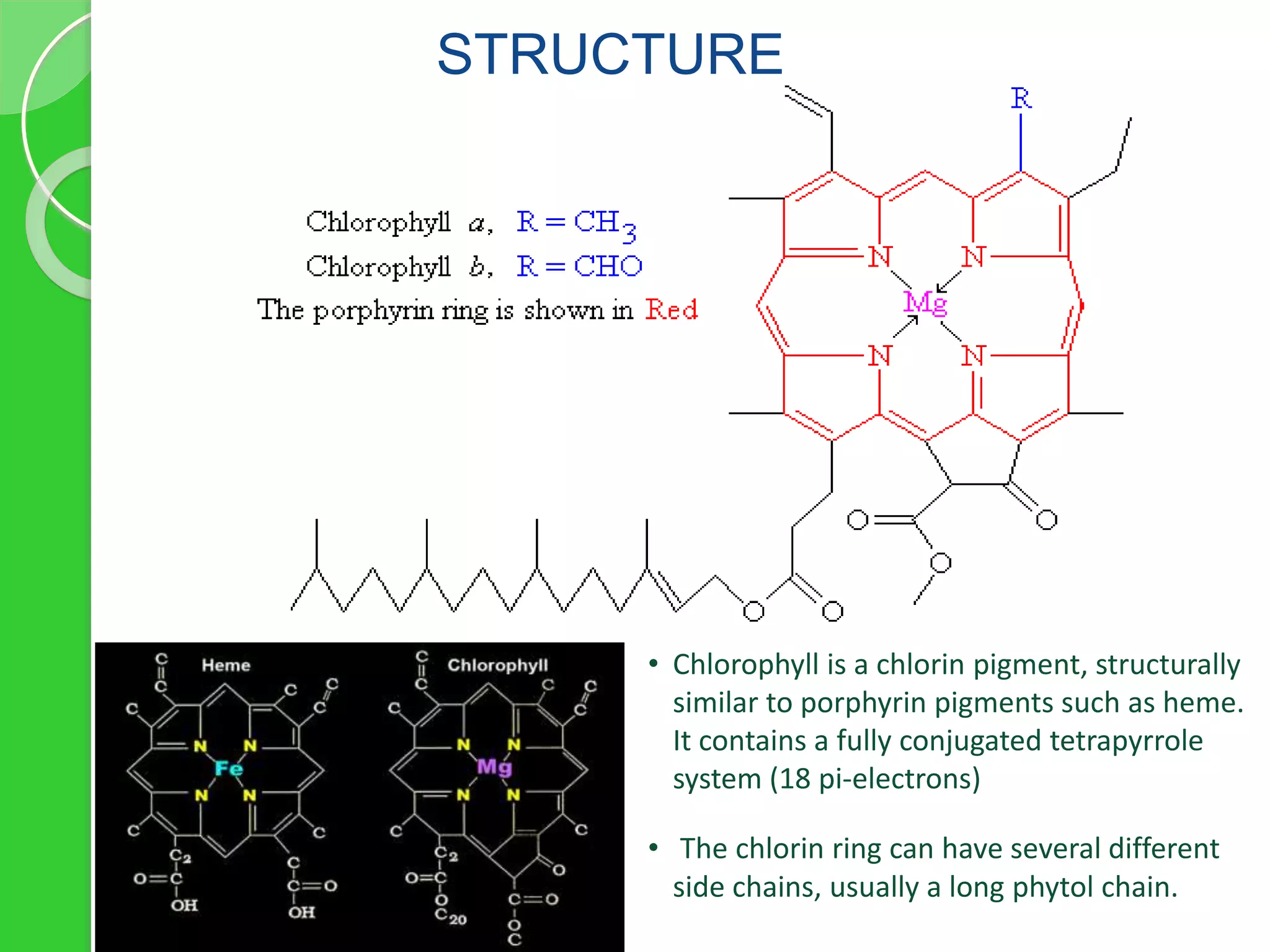
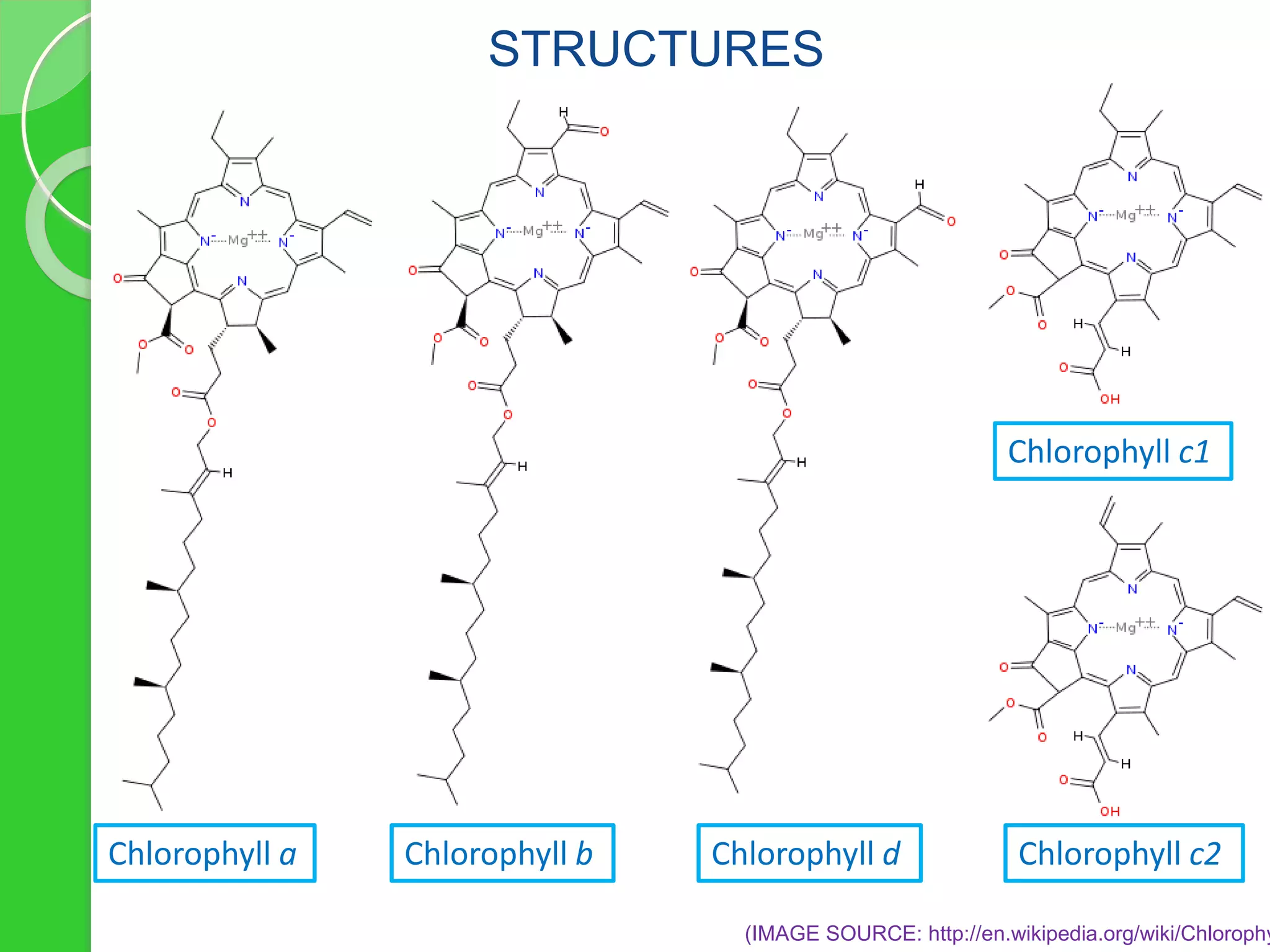
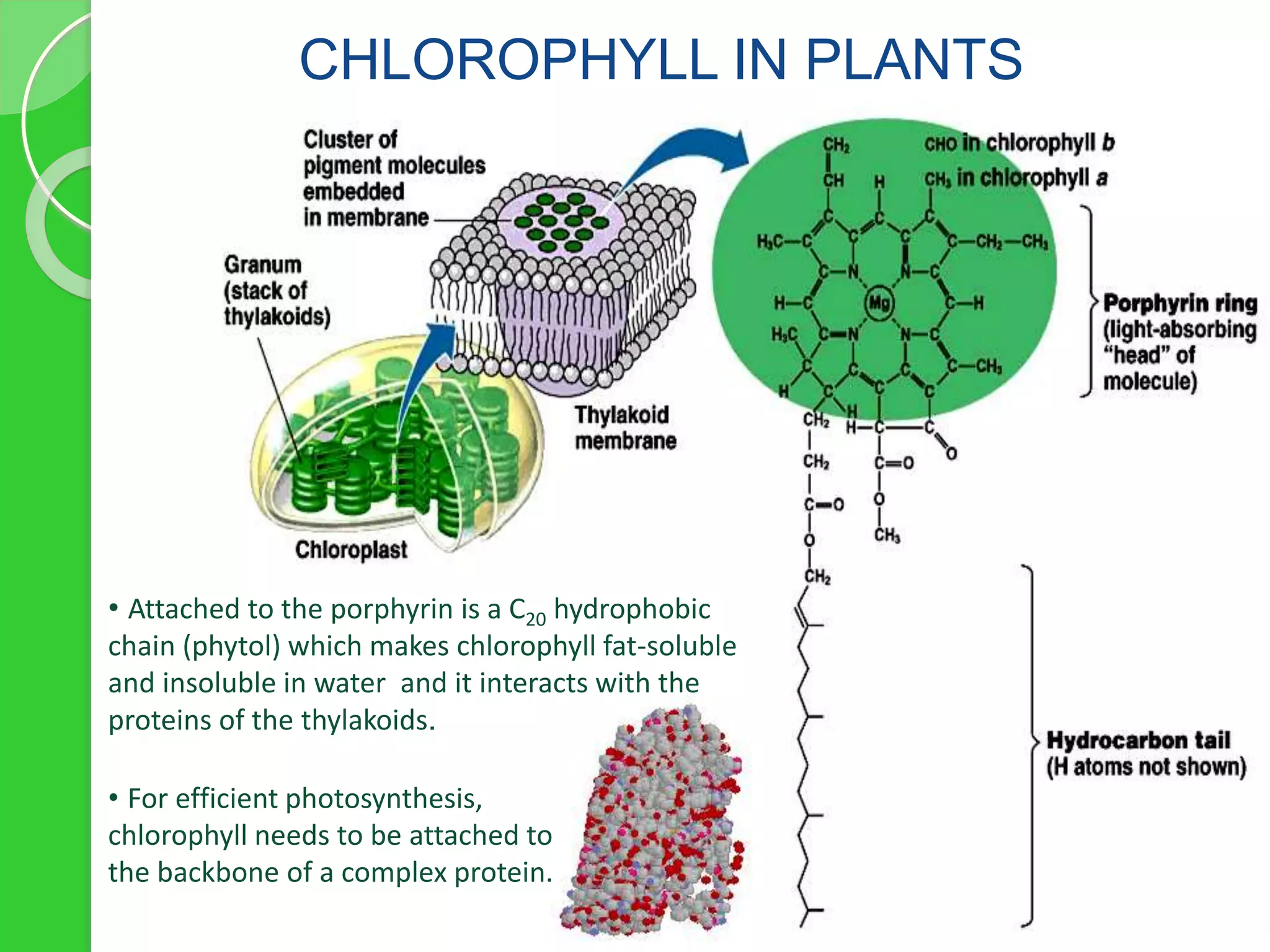
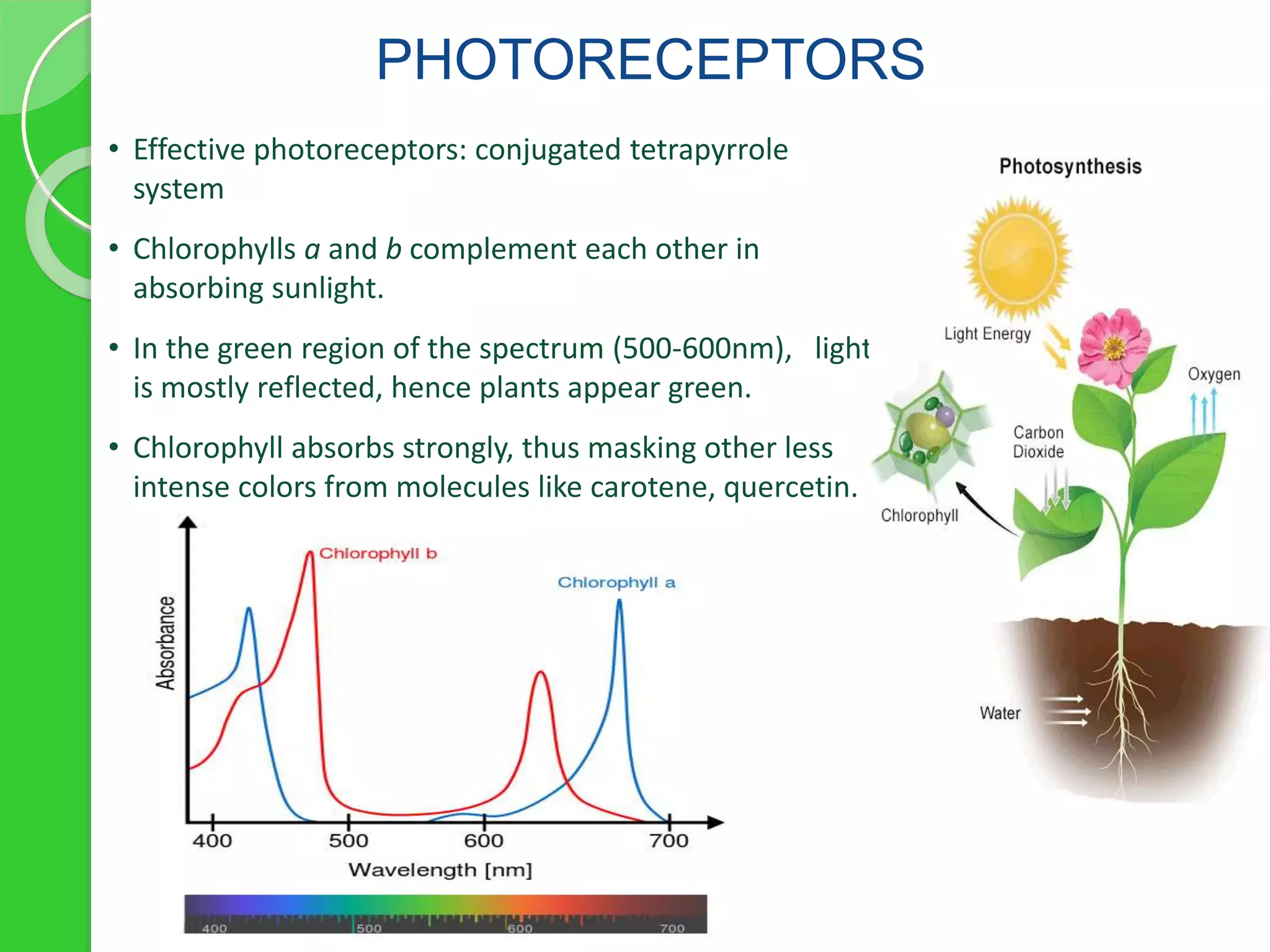
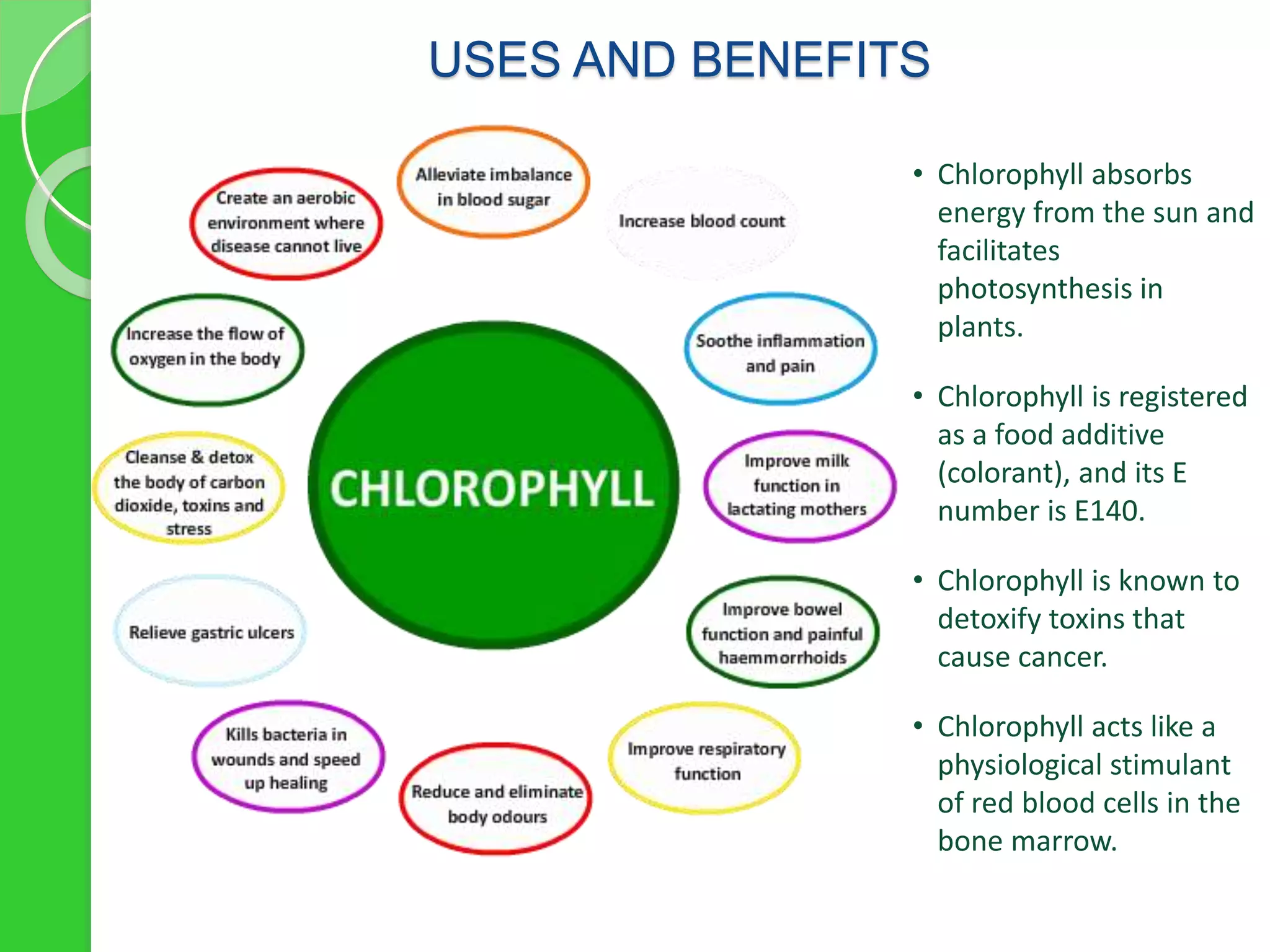
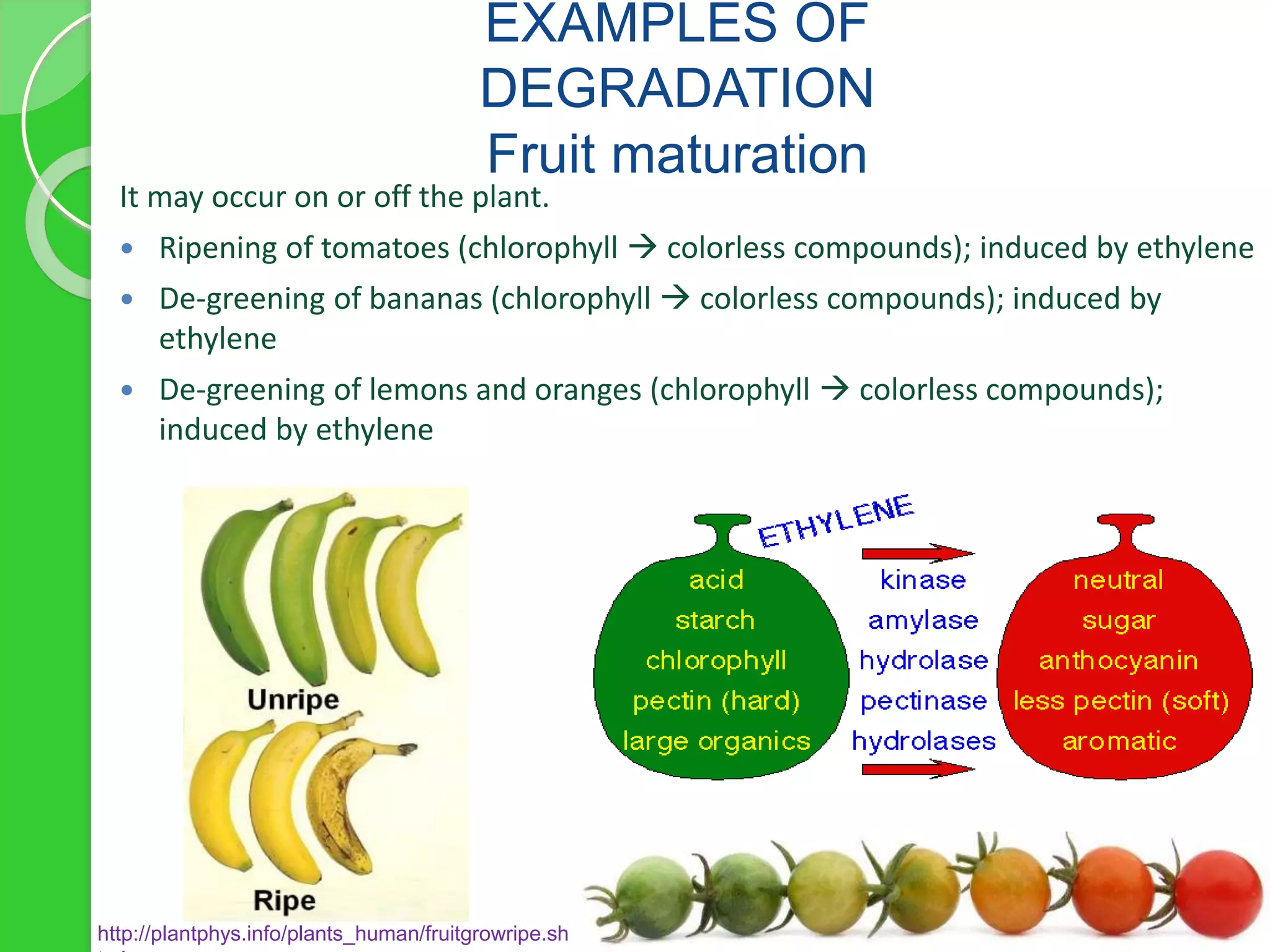
Chlorophyll is a crucial pigment for photosynthesis found in various plants and algae, primarily chlorophyll a and b, which have a specific structure that aids in absorbing sunlight. It has multiple uses, including acting as a food additive, a detoxifier, and a stimulant for red blood cell production. The stability and degradation of chlorophyll can be affected by factors such as pH and heat, and it can be preserved through methods like freezing and blanching.




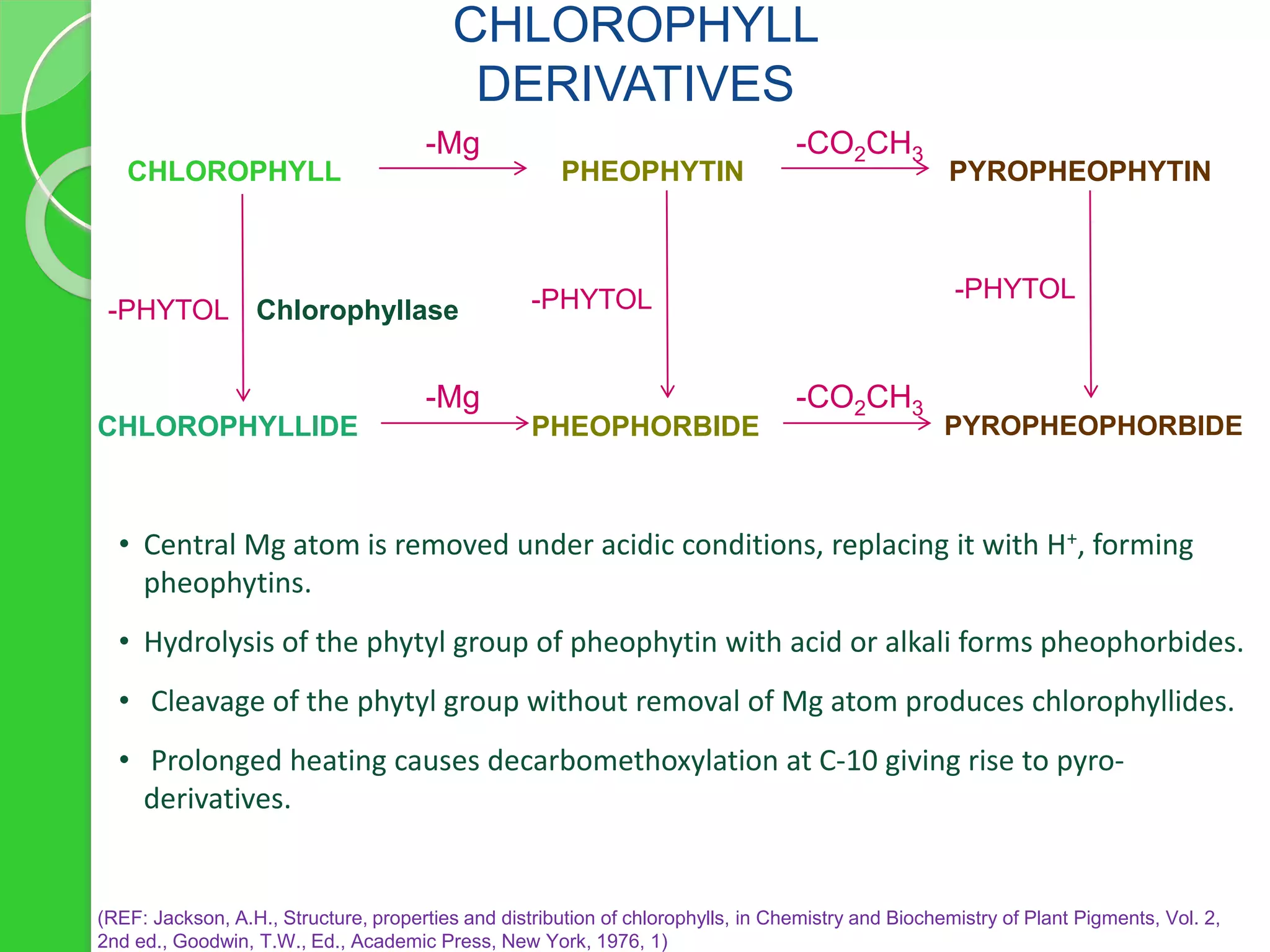
![ Canning of peas [chlorophyll pheophytin (olive brown)]; induced by heat
Brining of olives [chlorophyll pheophytin (olive brown) pheophorbide
(olive brown) and chlorophyll chlorophyllide (blue green)
pheophorbide]; induced by acid and chlorophyllase
Blanching of snap beans, turnip greens and okra[chlorophyll pheophytin
(olive brown)]; induced by heat
Coleslaw processing [chlorophyll pheophytin (olive brown)
pheophorbide (olive brown)]; induced by acid and chlorophyllase
PROCESSIN
G
(REF: Heaton, J.W. and Marangoni, A.G., Chlorophyll degradation in processed foods and senescent plant tissues, Trends Food Sci.](https://image.slidesharecdn.com/chlorophyll-180219174133/75/Chlorophyll-13-2048.jpg)