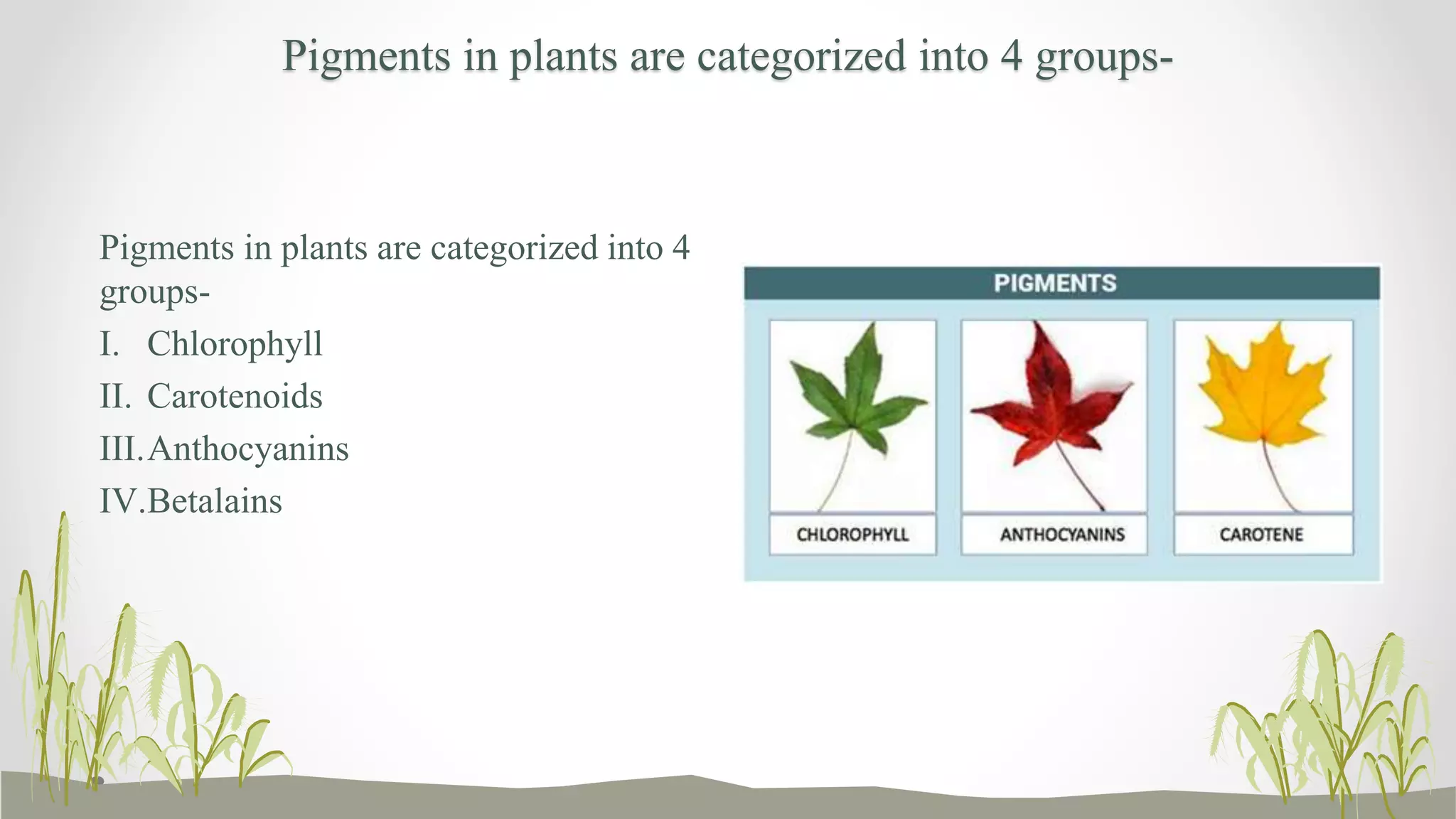
The document discusses plant pigments, emphasizing their roles, classifications, and utilities. It categorizes pigments into four groups: chlorophyll, carotenoids, anthocyanins, and betalains, each serving vital functions in plant physiology and reproduction. Additionally, it explores value-added products derived from these pigments, highlighting their applications in food, beverages, and the fragrance industry.