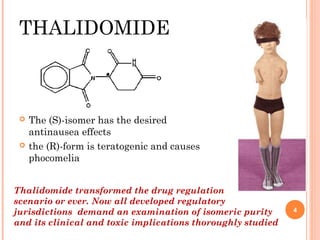
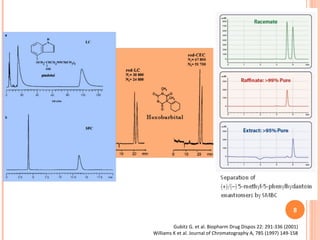
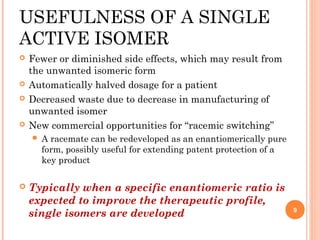
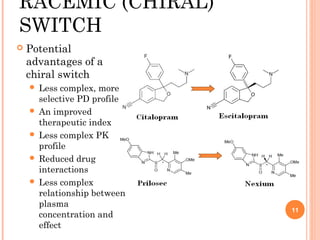
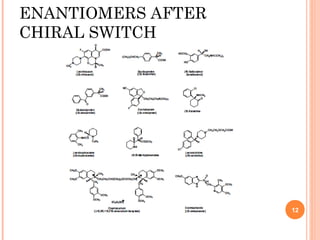
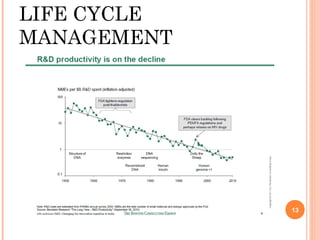
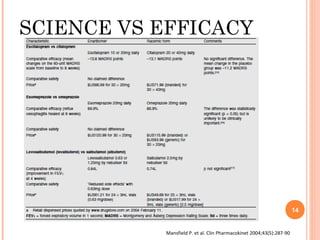
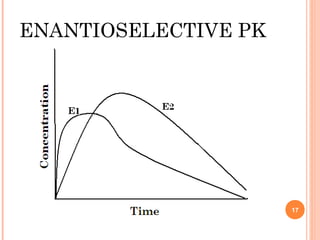
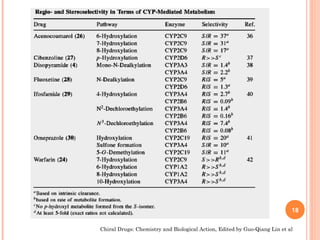
This document summarizes a presentation on chiral separation from a pharmaceutical industry perspective. It begins by discussing the tragic lessons from Thalidomide, where one enantiomer was teratogenic while the other had desired effects. It emphasizes the importance of separating single enantiomers due to differences in biological activity and toxicity. It then covers topics like chiral stationary phases used for separation, potential advantages of racemic switches and developing single isomers, and considerations for preclinical and clinical development like enantioselective assays and pharmacokinetics. In concluding, it stresses that isomeric purity must be thoroughly studied and chiral switches should improve safety/efficacy.