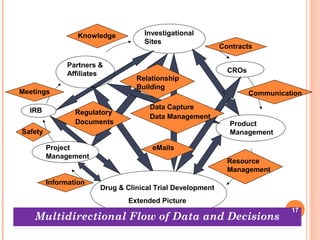
The document discusses the importance of ethics in the pharmacy profession and clinical research, emphasizing moral principles such as respect for individuals, informed consent, and data integrity. It outlines responsibilities for pharmacists towards consumers, the community, and their profession, along with ethical guidelines for biomedical research on human subjects. The document highlights the complexities of ethical considerations in clinical trials and the significance of maintaining data integrity throughout the research process.