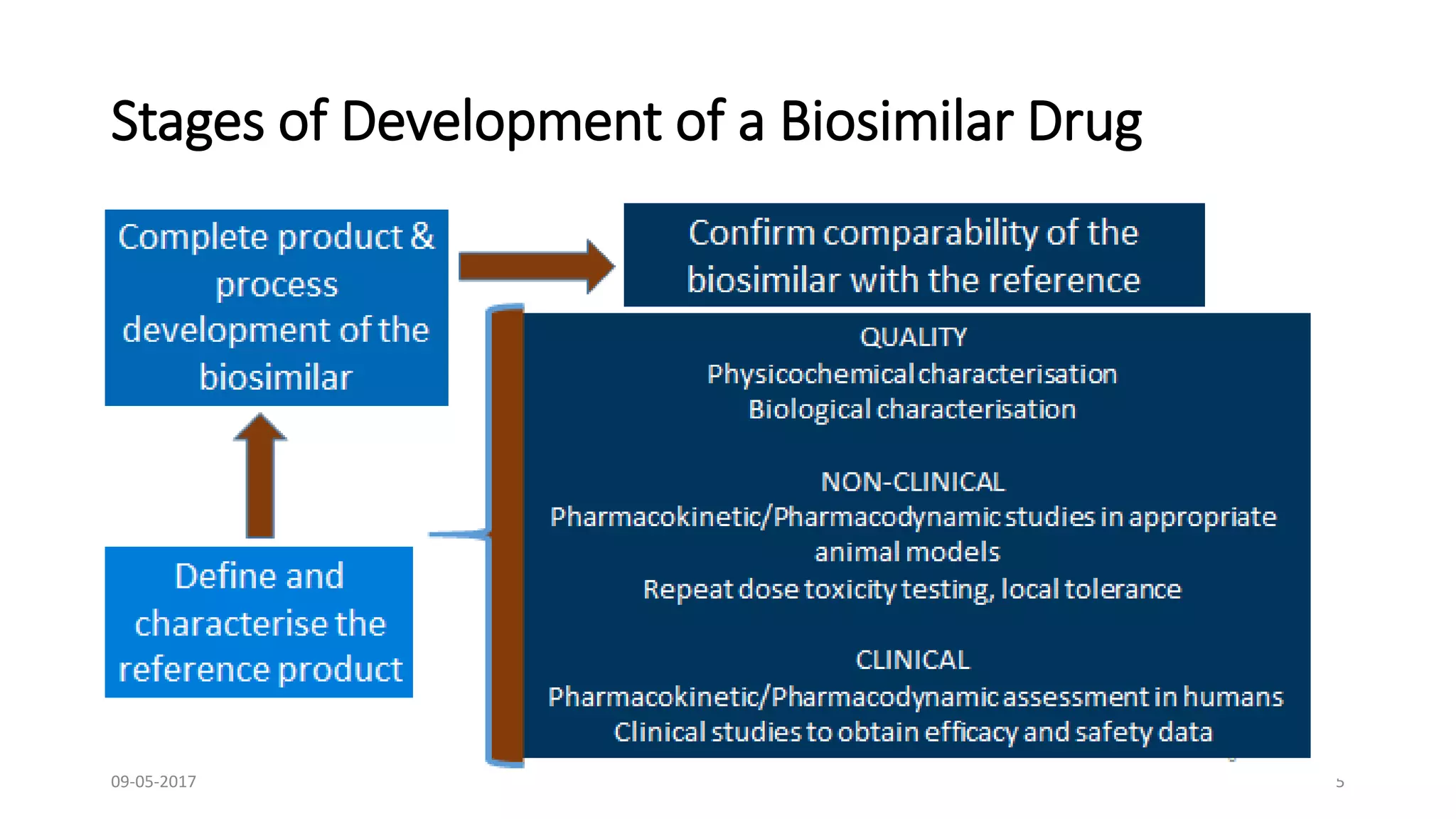
The document presents a comprehensive overview of the principles and processes involved in the clinical development of biosimilars, as discussed by Dr. Bhaswat S. Chakraborty at a national conference. It covers essential aspects such as the assessment of biosimilarity, immunogenicity, and the regulatory requirements for biosimilars in terms of safety, efficacy, and quality. Additionally, it emphasizes the importance of a totality-of-evidence approach in demonstrating biosimilarity and the challenges related to heterogeneity and comparability assessments.



































![Post-approval Commitment [example]
3809-05-2017](https://image.slidesharecdn.com/clinicaldevelopmentofbiosimilarsfinal-170509043713/75/Clinical-Development-of-Biosimilars-37-2048.jpg)


