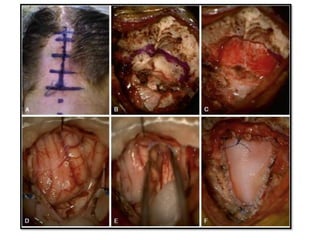
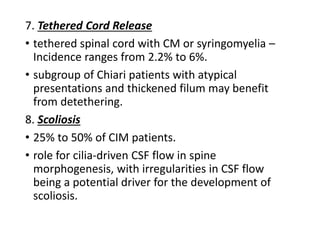
Chiari malformations are a collection of hindbrain abnormalities characterized by cerebellar herniation and varying clinical presentations. Treatment focuses on restoring cerebrospinal fluid dynamics, particularly in symptomatic patients, with surgical decompression recommended within two years of symptom onset. Types I and II Chiari malformations are most prevalent, with Type II often associated with myelomeningocele and more severe symptoms.