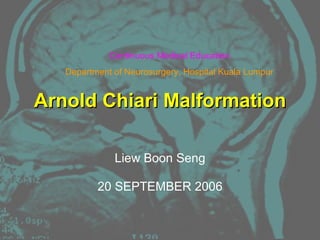
Arnold Chiari malformation is a group of congenital anomalies affecting the posterior fossa. There are four types of Chiari malformation classified based on the degree and contents of herniation through the foramen magnum. Type I involves herniation of the cerebellar tonsils, while Type II involves herniation of the cerebellar tonsils and contents of the posterior fossa into the foramen magnum. Posterior fossa decompression is the main surgical treatment, involving enlarging the posterior fossa volume and establishing CSF flow, to relieve compression of neural structures. Complications can include CSF leaks, recurrent syringomyelia, and persistent neurological deficits.