The document outlines a procedure for analyzing unknown cations using a systematic separation scheme, detailing groupings of ions and necessary reagents. It emphasizes safety precautions and the importance of pH adjustment while conducting tests for various cations. Key instructions include using specific chemical solutions and observing precipitate formations to confirm the presence of certain ions.


























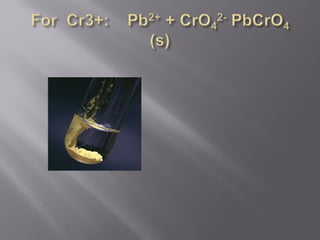





![For instance
Ksp = [Pb2+][CRO4 2-] =
The group ii cations Bi3+ , Cu 2+ , Cd 2+ and Sn
4+
Form insoluble sulfide precipitates even at very
low sulfide ion (S 2-) concentrations.](https://image.slidesharecdn.com/qualitativegroupiiinewthisone-121123104349-phpapp02/85/Qualitativegroup-iiine-wthis-one-33-320.jpg)
