These documents describe 10 experiments analyzing unknown salts to identify their acidic and basic radicals. Each experiment provides observations of preliminary tests and acid-base radical tests. The results identify the following ion pairs:
1. Ammonium (NH4+) and acetate (CO32-);
2. Ammonium (NH4+) and acetate (CH3COO-);
3. Ammonium (NH4+) and chloride (Cl-);
4. Lead (Pb2+) and nitrate (NO3-);
5. Copper (Cu2+) and sulphate (SO42-);
6. Aluminium (Al3+) and sulphate (SO42-



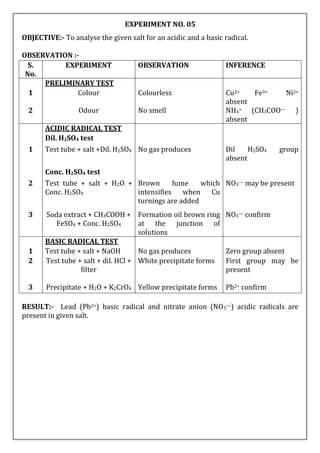
![EXPERIMENT NO. - 06
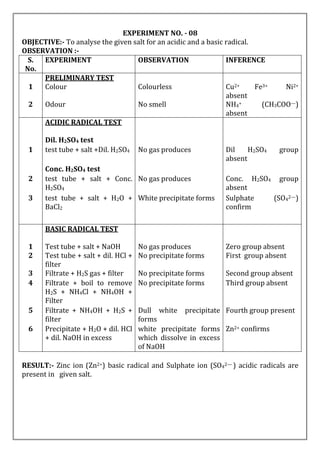
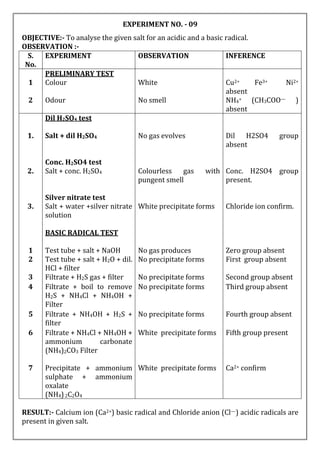
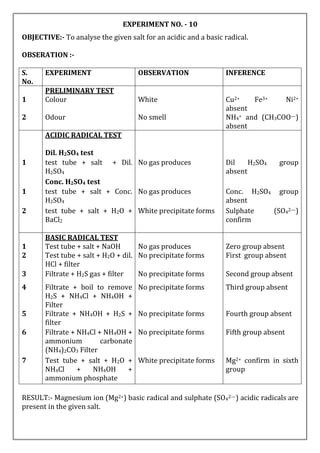
OBJECTIVE:- To analyse the given salt for an acidic and a basic radical.
OBSERVATION :-
S.
No.
EXPERIMENT OBSERVATION INFERENCE
PRELIMINARY TEST
1 Colour Blue colour Cu2+ may be presnt
2 Odour No smell NH4+ (CH3COO—)
absent
ACIDIC RADICAL TEST
Dil. H2SO4 test
1 test tube + salt +Dil. H2SO4 No gas produces Dil H2SO4 group
absent
Conc. H2SO4 test
2 test tube + salt + Conc.
H2SO4
No gas produces Conc. H2SO4 group
absent
3 test tube + salt + H2O +
BaCl2
White precipitate forms Sulphate (SO42—)
confirm
BASIC RADICAL TEST
1 Test tube + salt + NaOH No gas producses Zero group absent
2 Test tube + salt + dil. HCl +
filter
No precipitate forms First group absent
3 Filtrate + H2S gas + filter Black Precipitate forms Second group present
4 Precipitate + Dil. HNO3 +
acetic acid + K4[Fe(CN)6]
Chocolate brown
coloured precipitate
forms
Cu2+ confirm
RESULT:- Cupric ion (Cu2+ )basic radical and Sulphate anion (SO42— ) acidic radicals
are present in given salt.](https://image.slidesharecdn.com/02-230527024209-c4ea2515/85/02-SALT-ANALYSIS-docx-5-320.jpg)