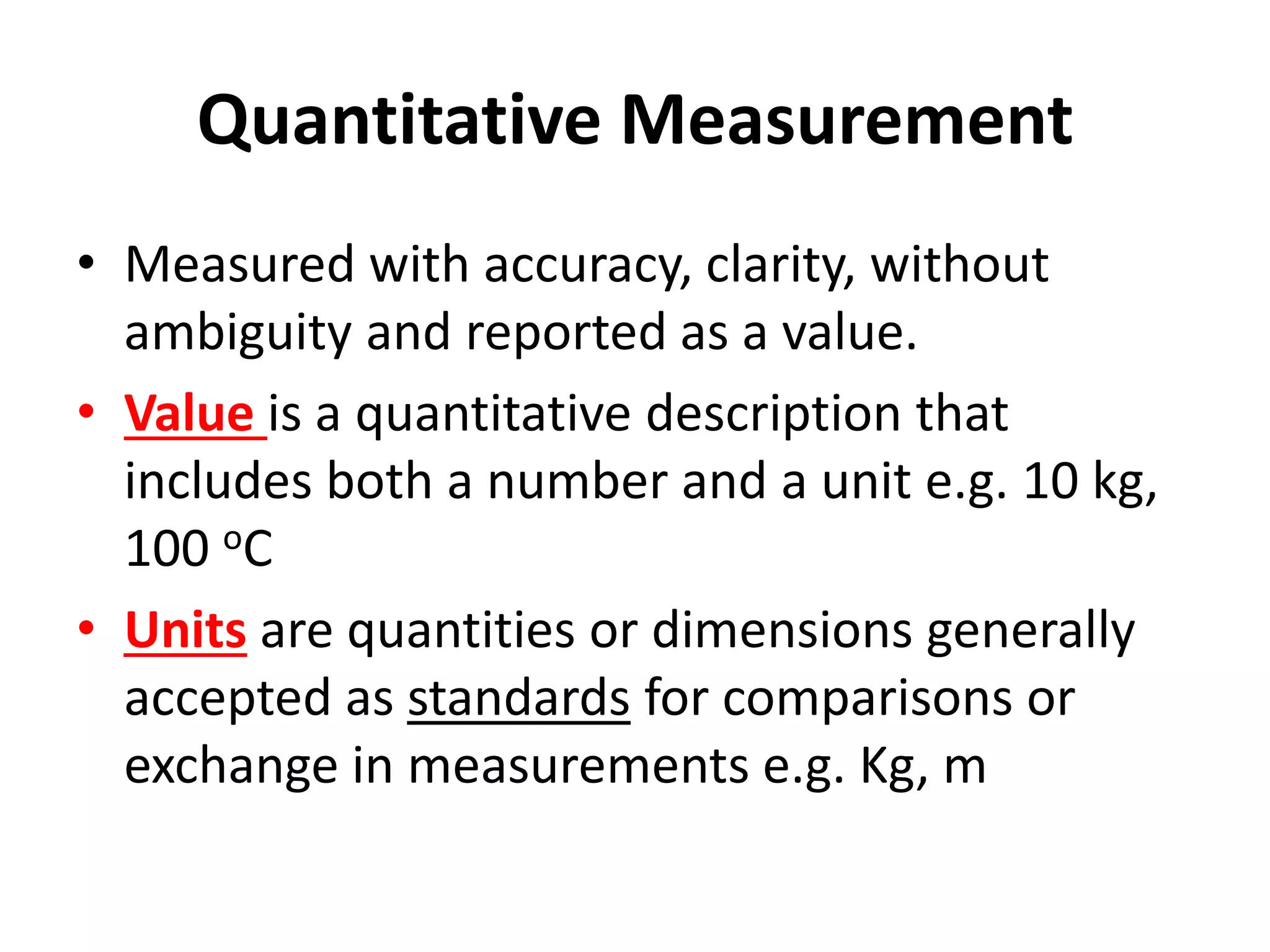
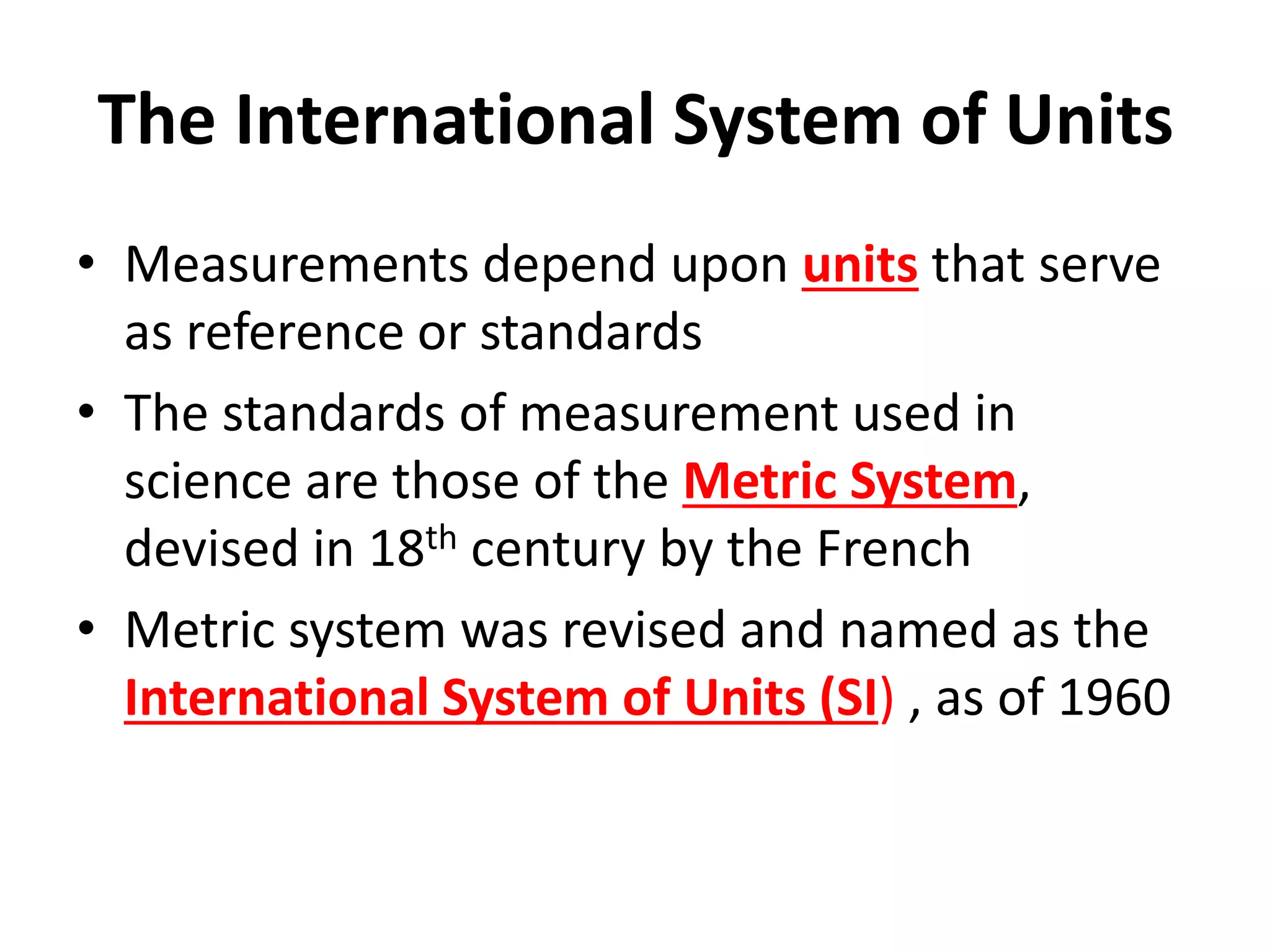
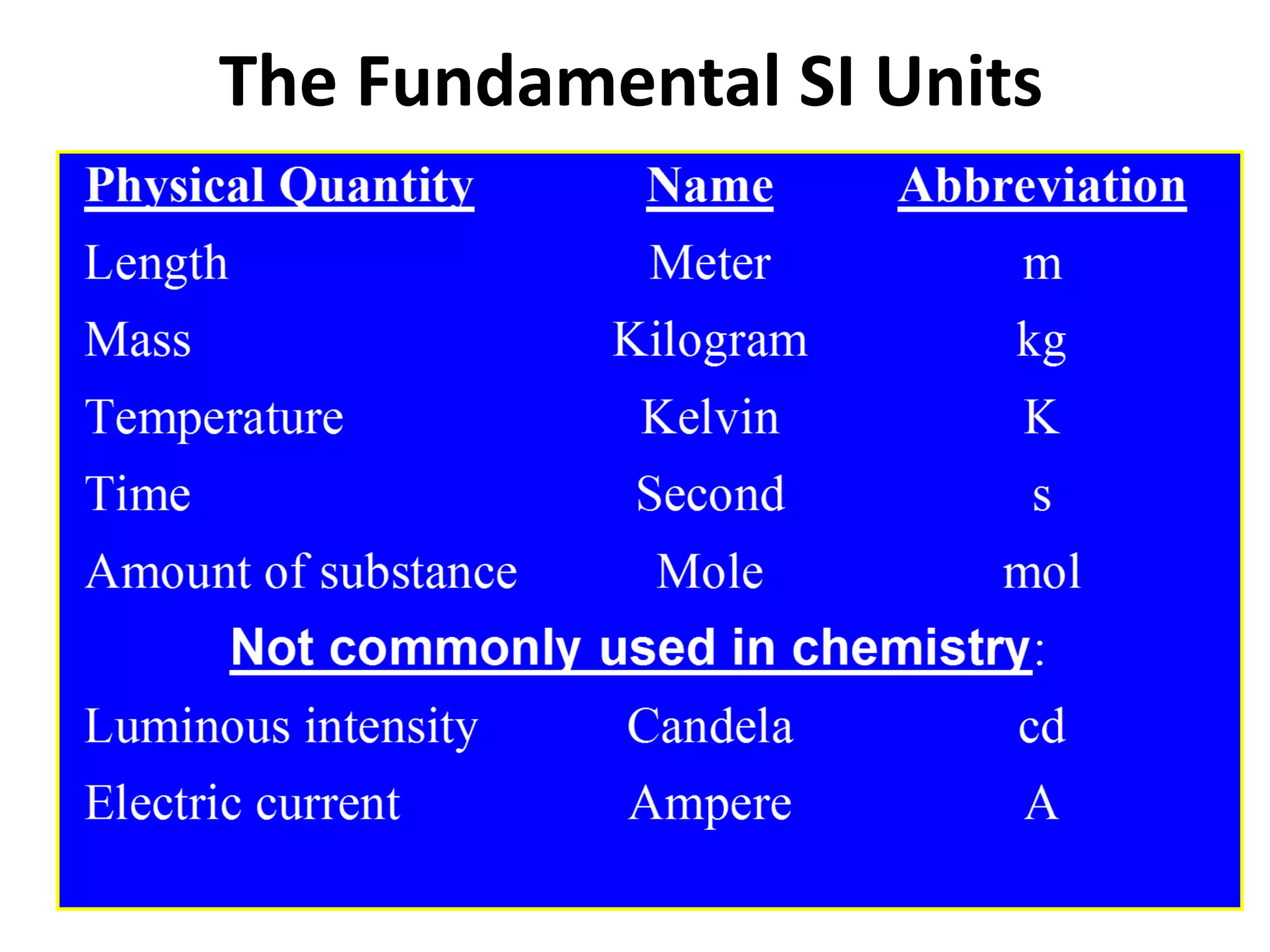
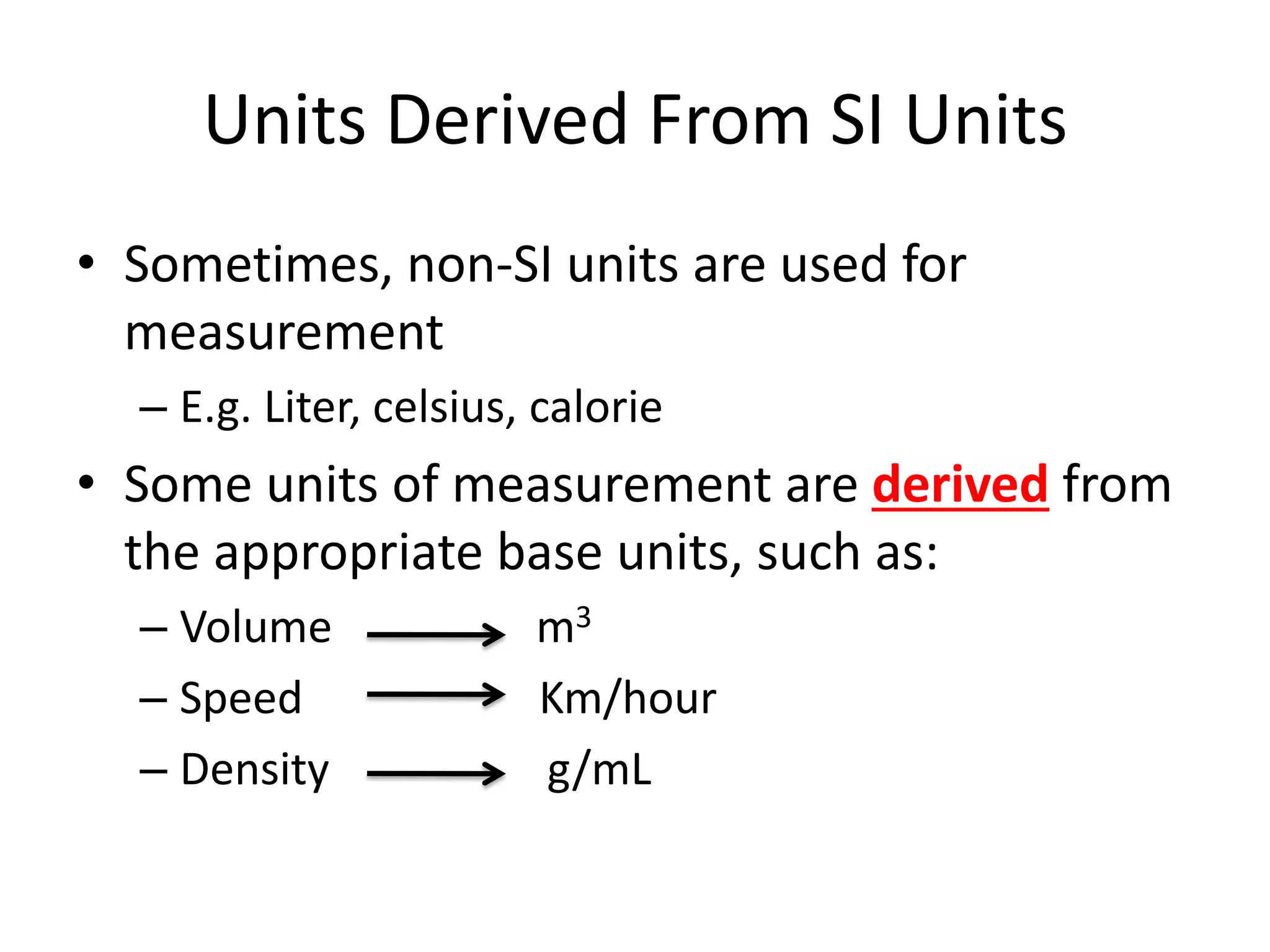
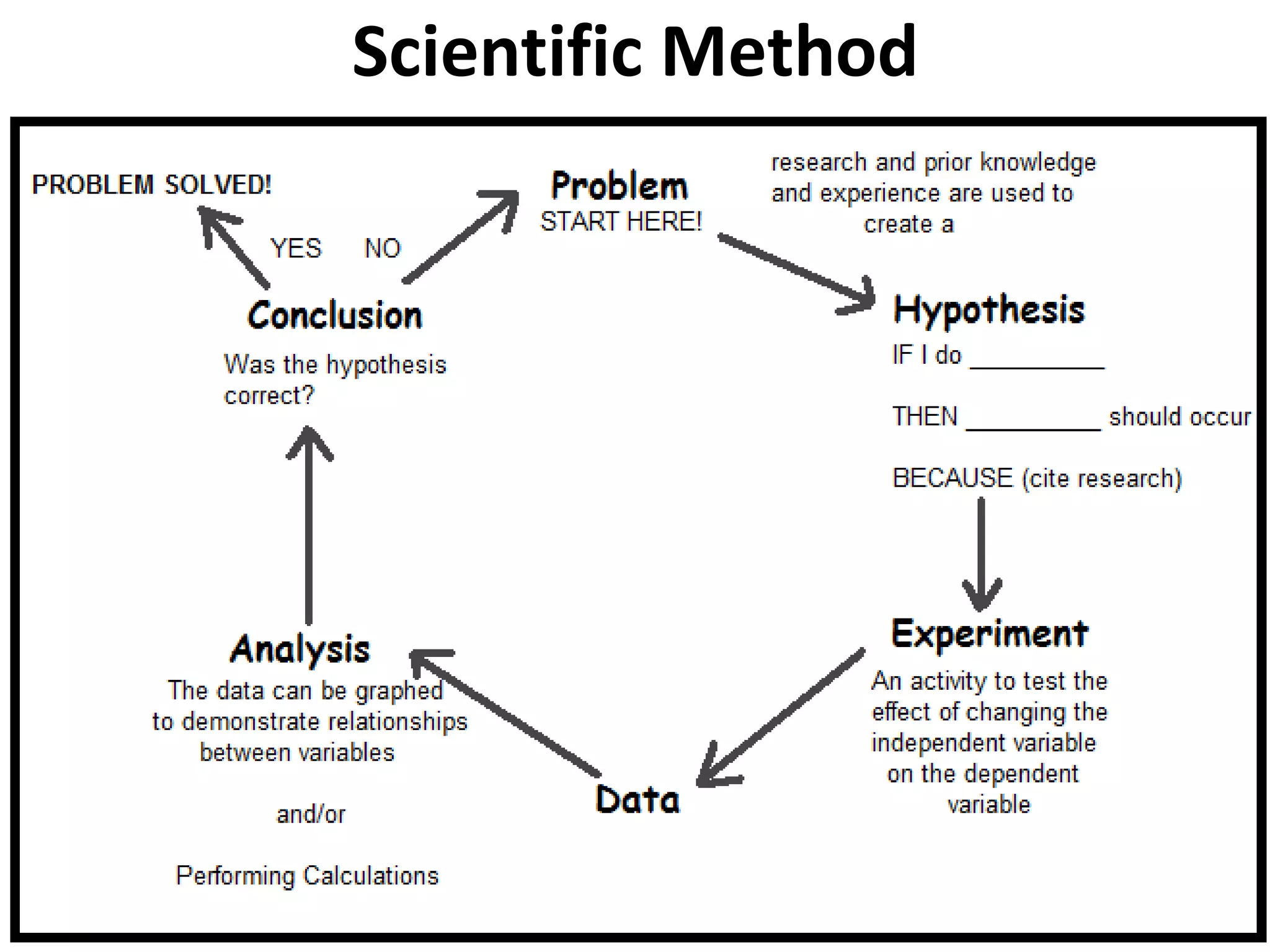
This document outlines the key topics covered in a course on scientific measurement:
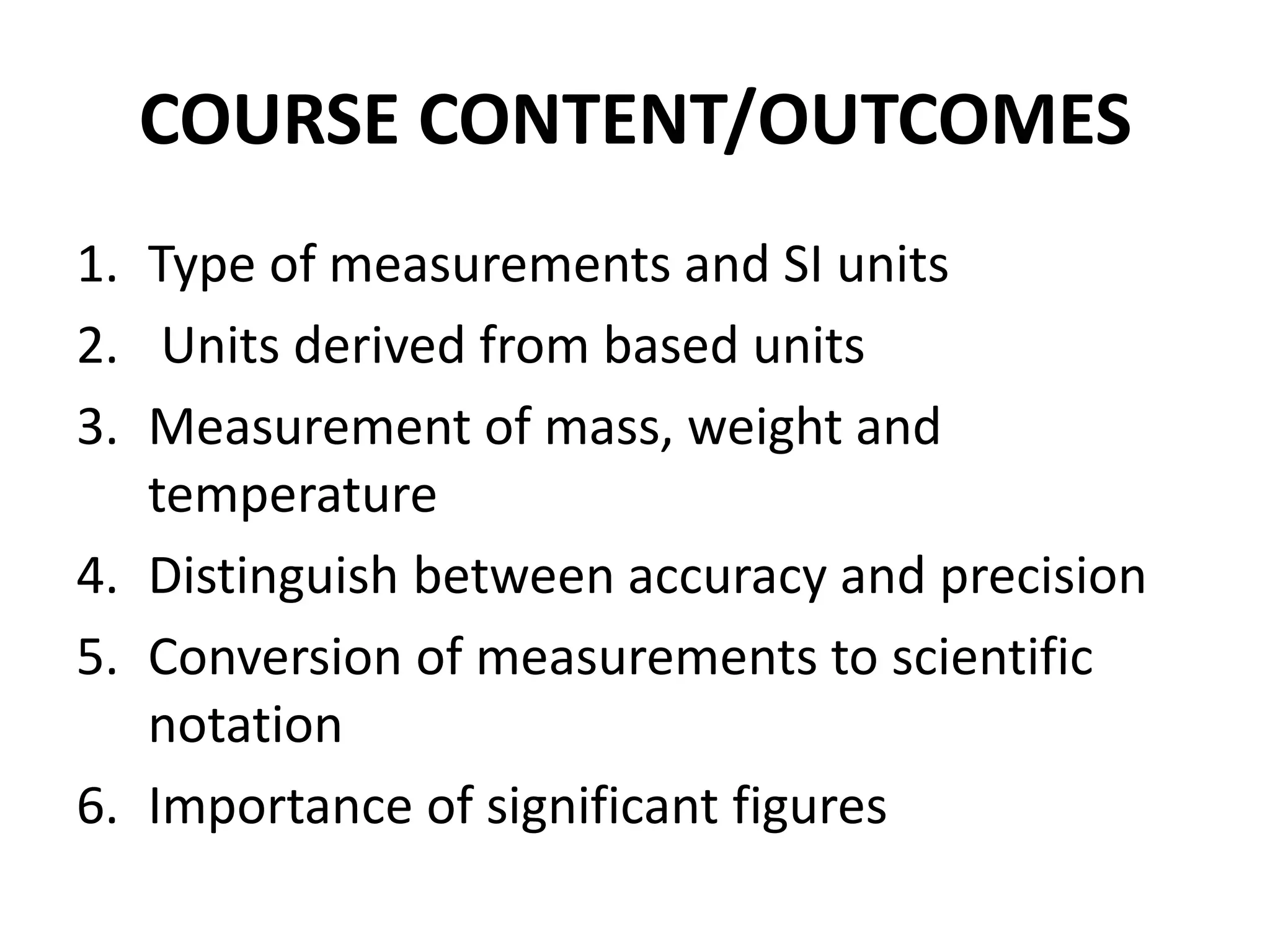
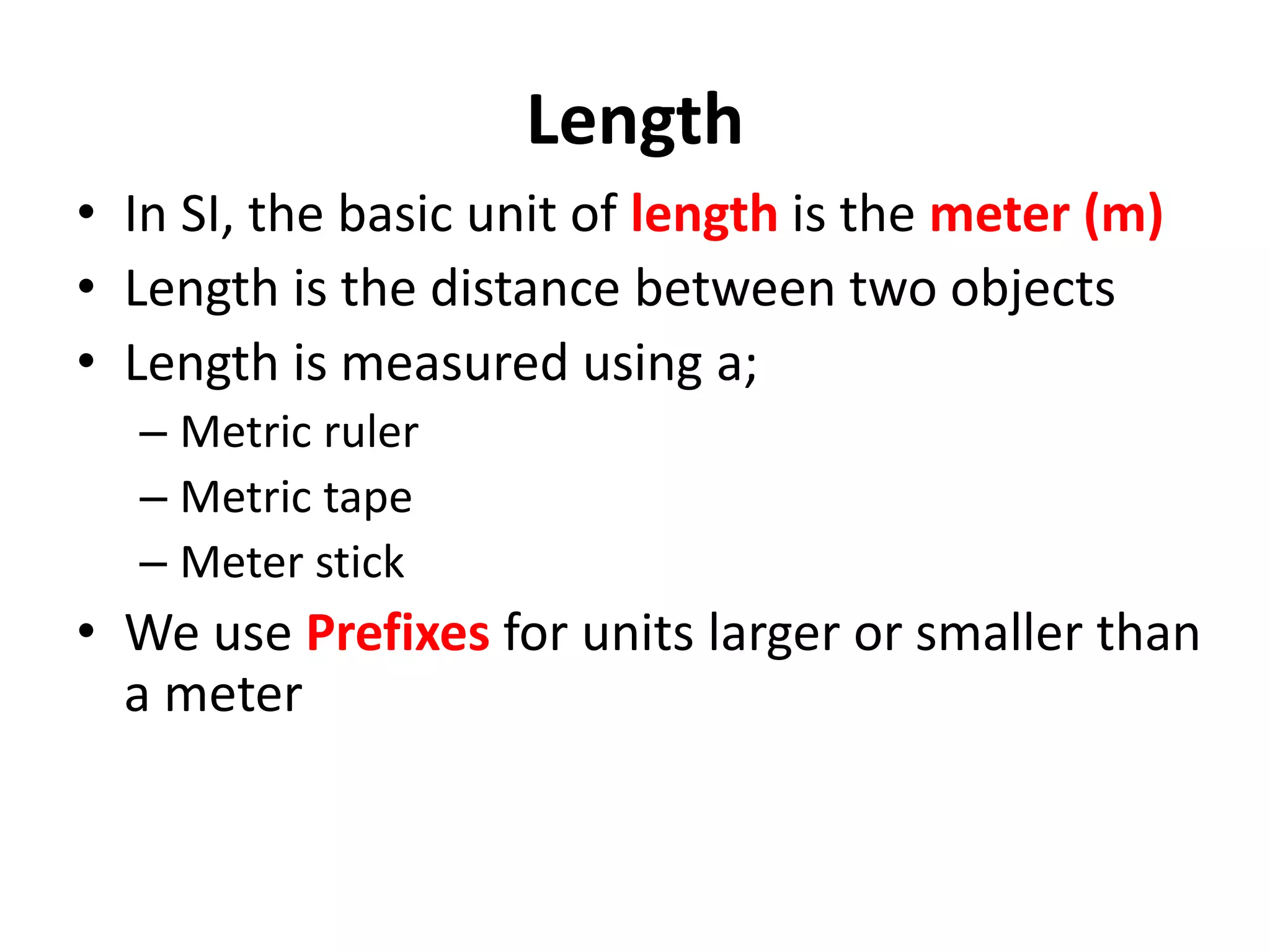
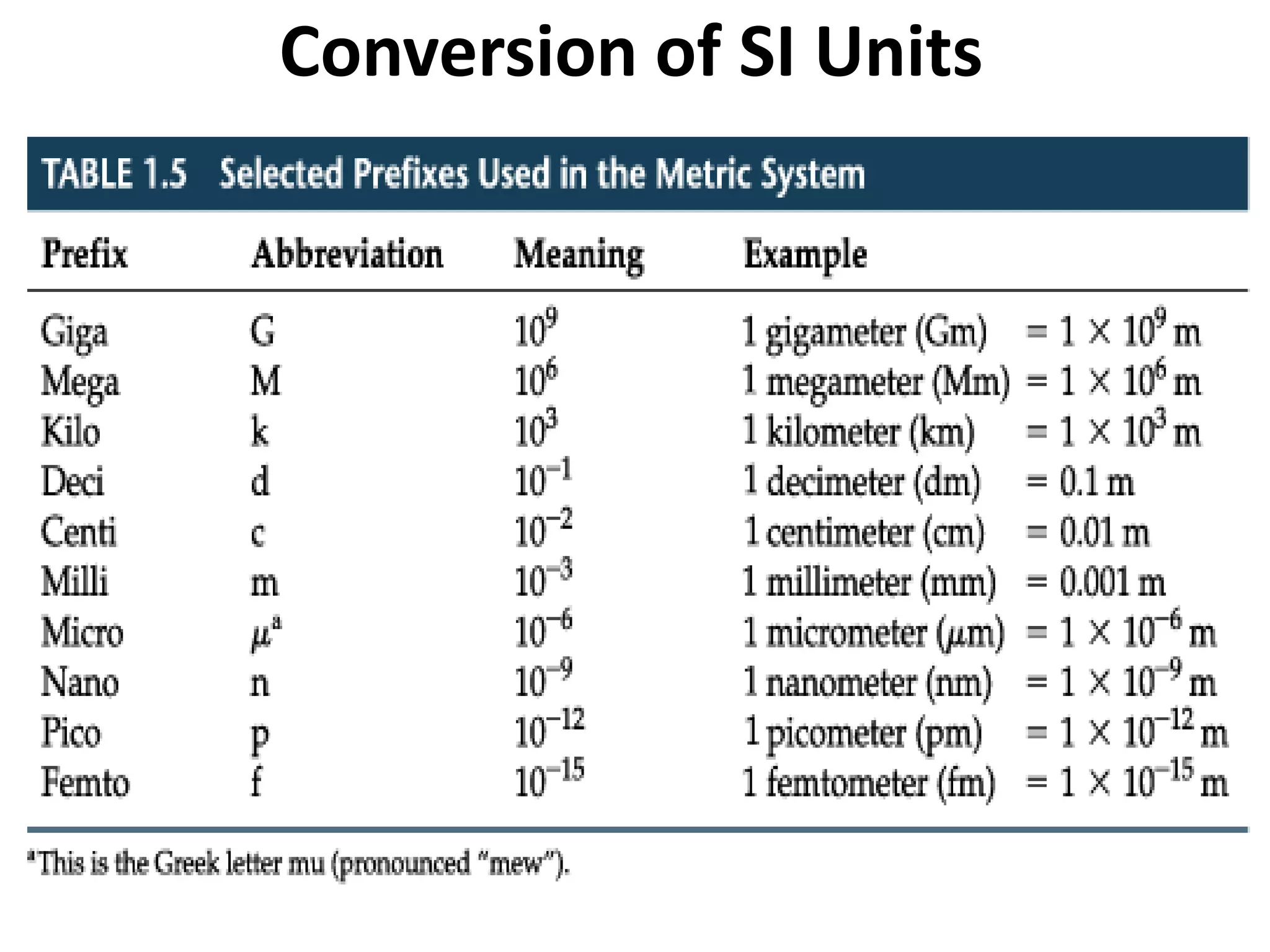
1. It introduces different types of measurements and SI units.
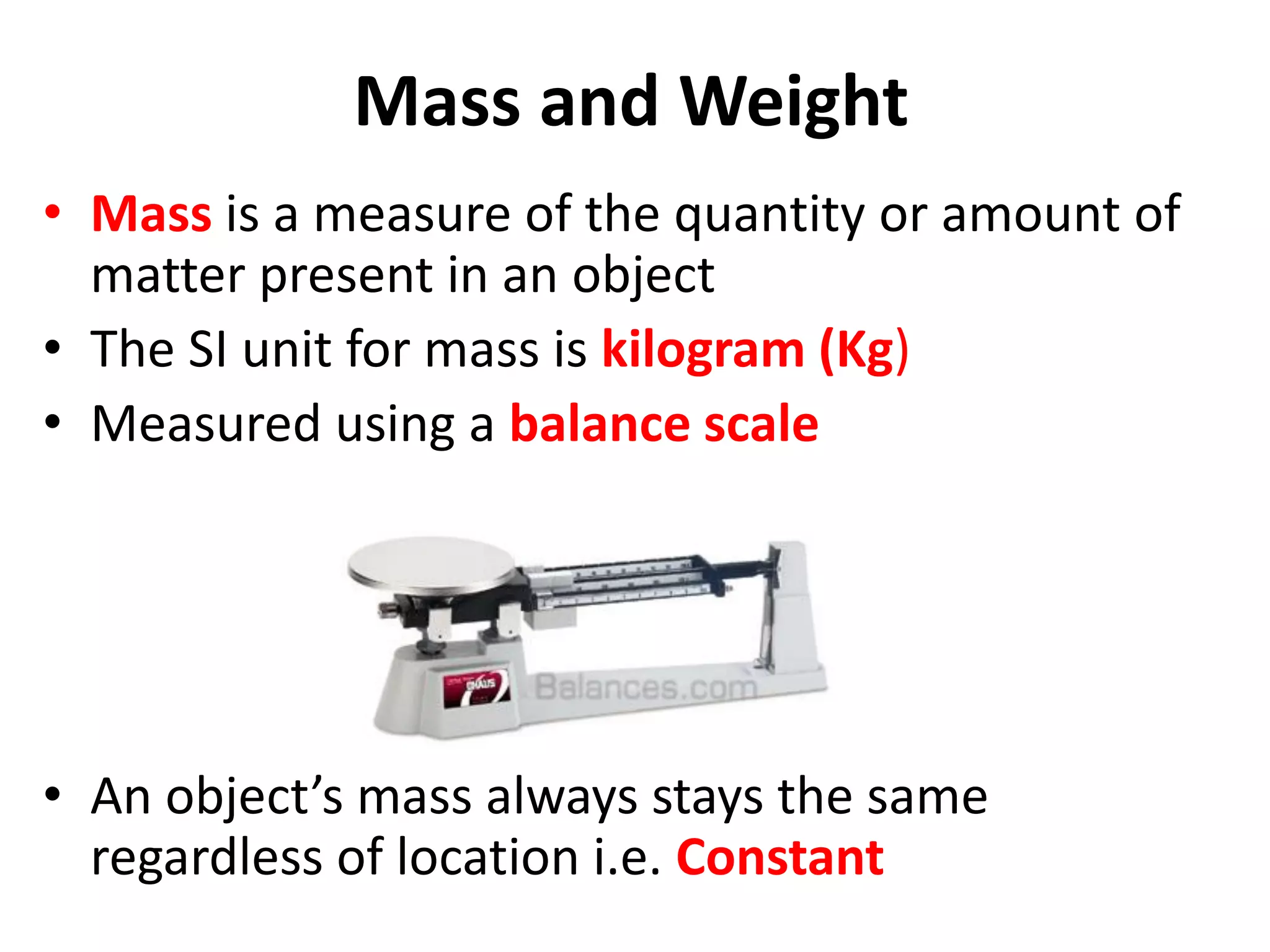
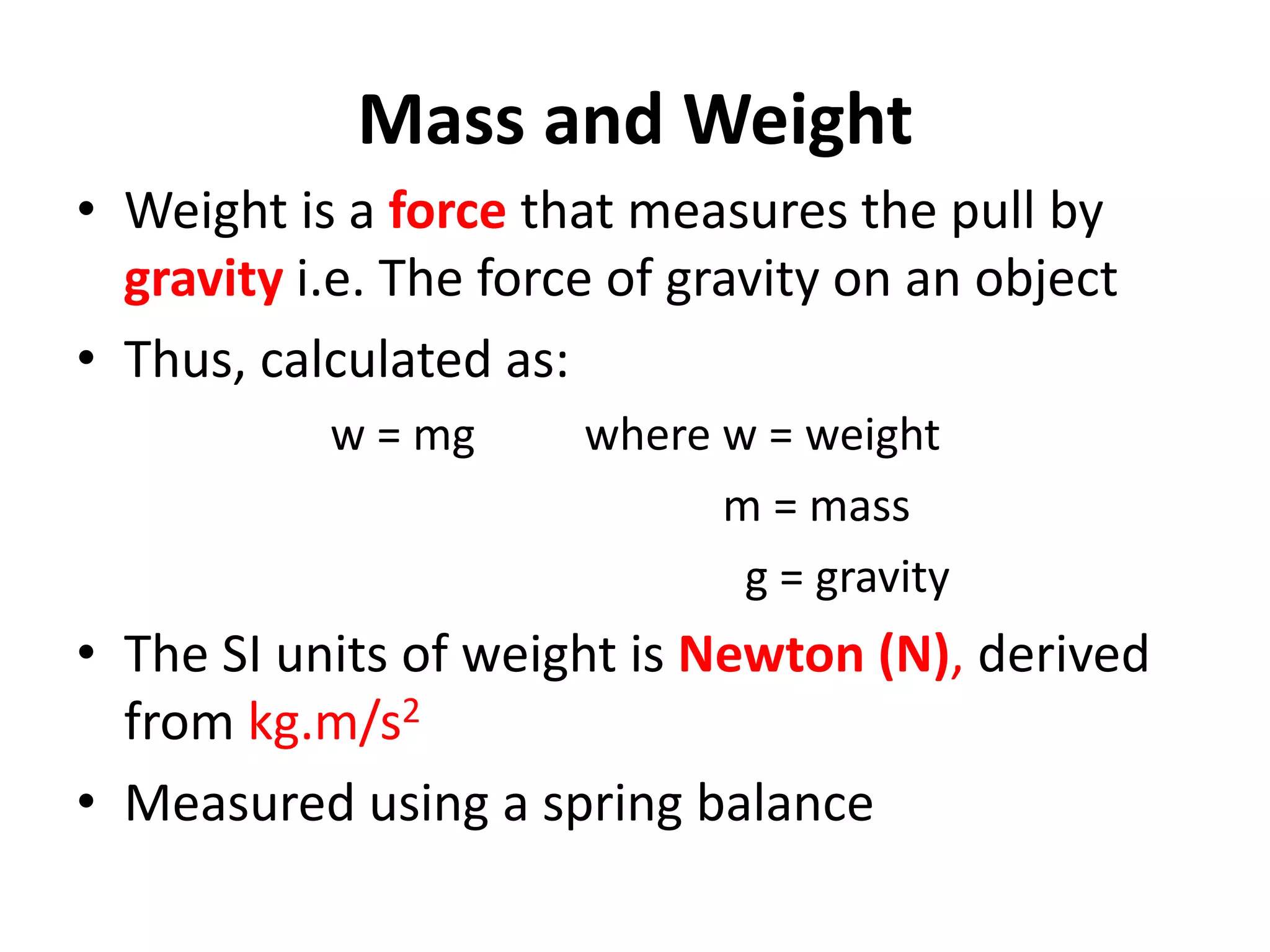
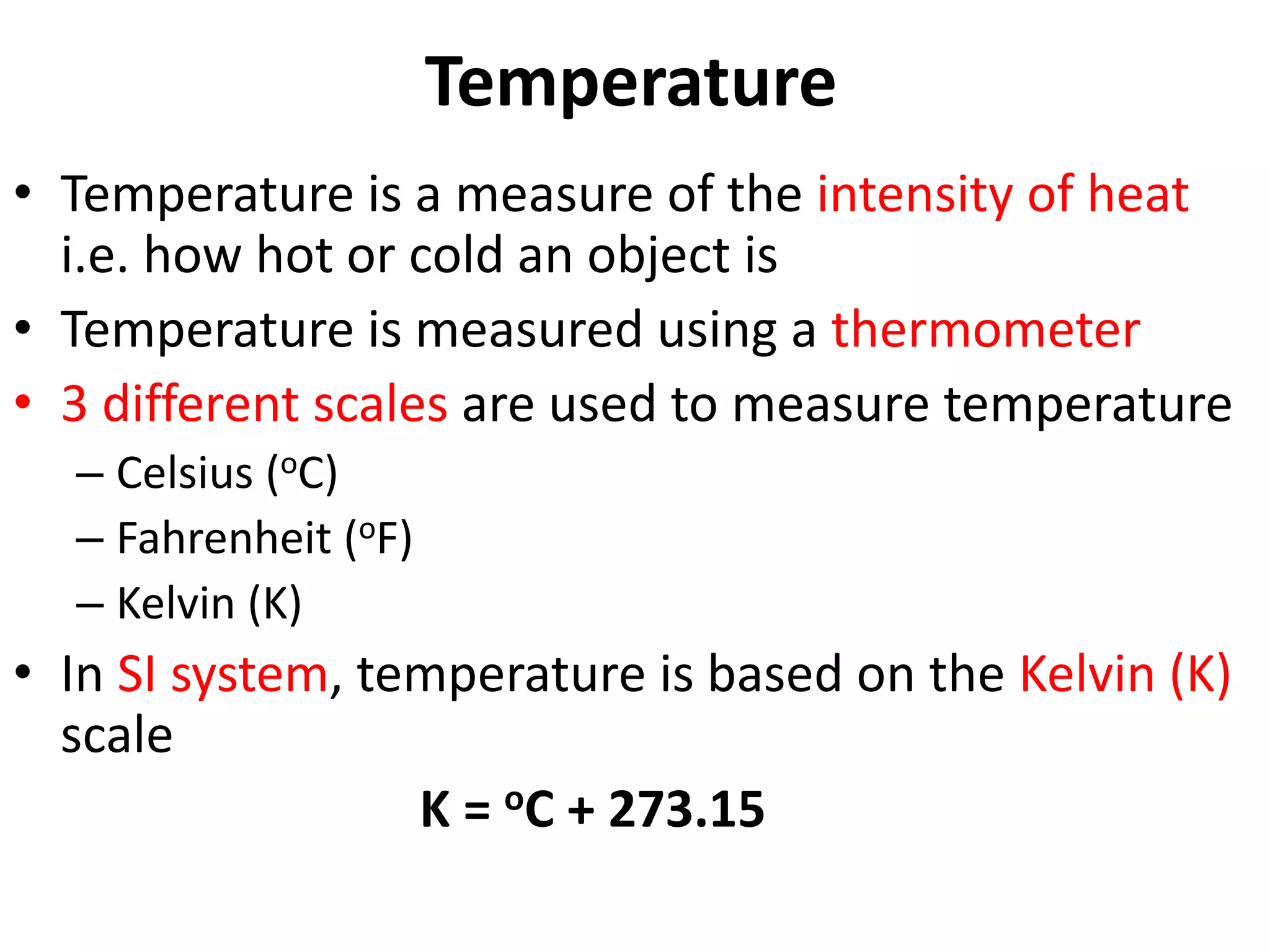
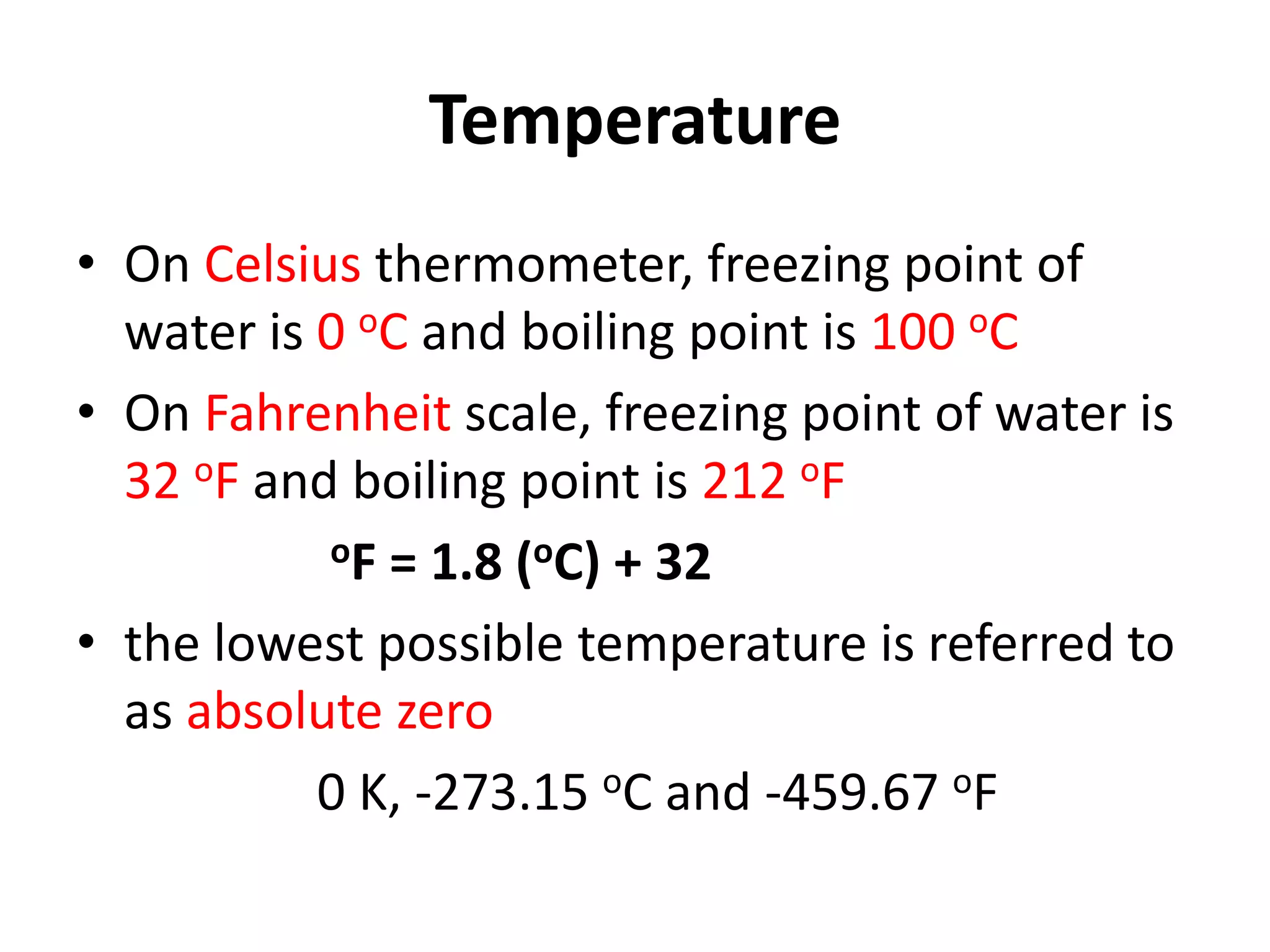
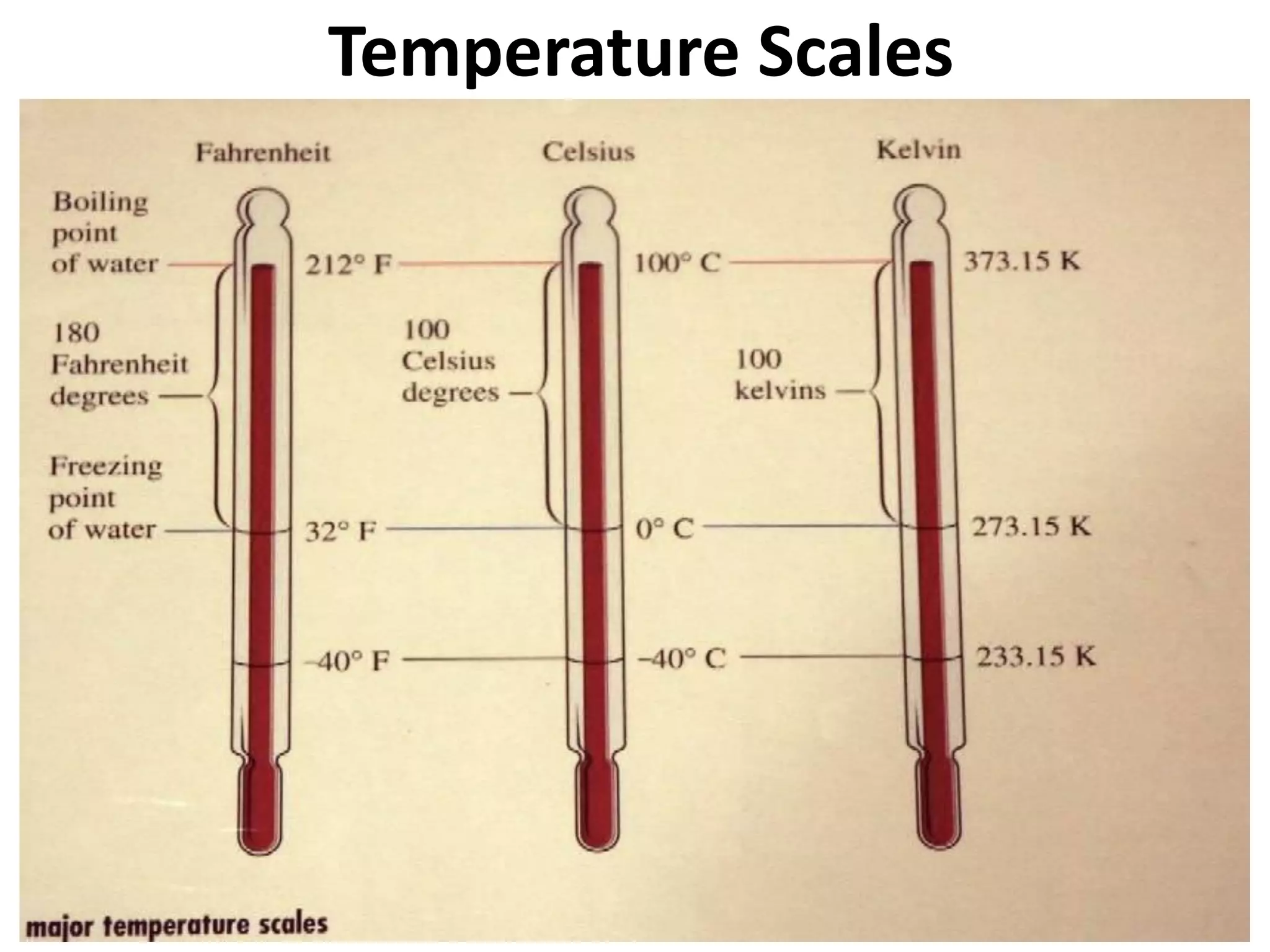
2. It discusses derived units, and measuring mass, weight, and temperature.
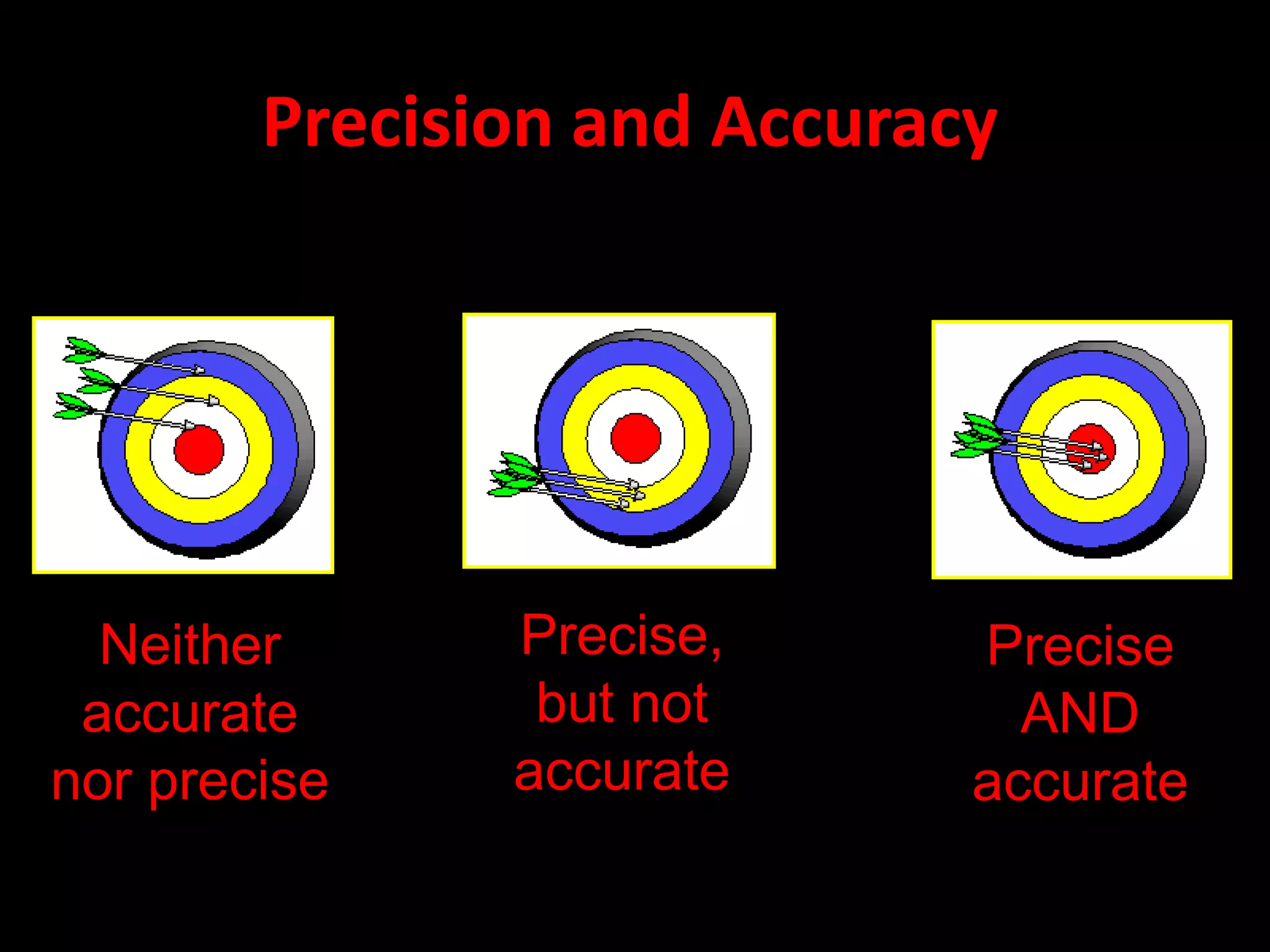
3. It distinguishes between accuracy and precision in measurements.
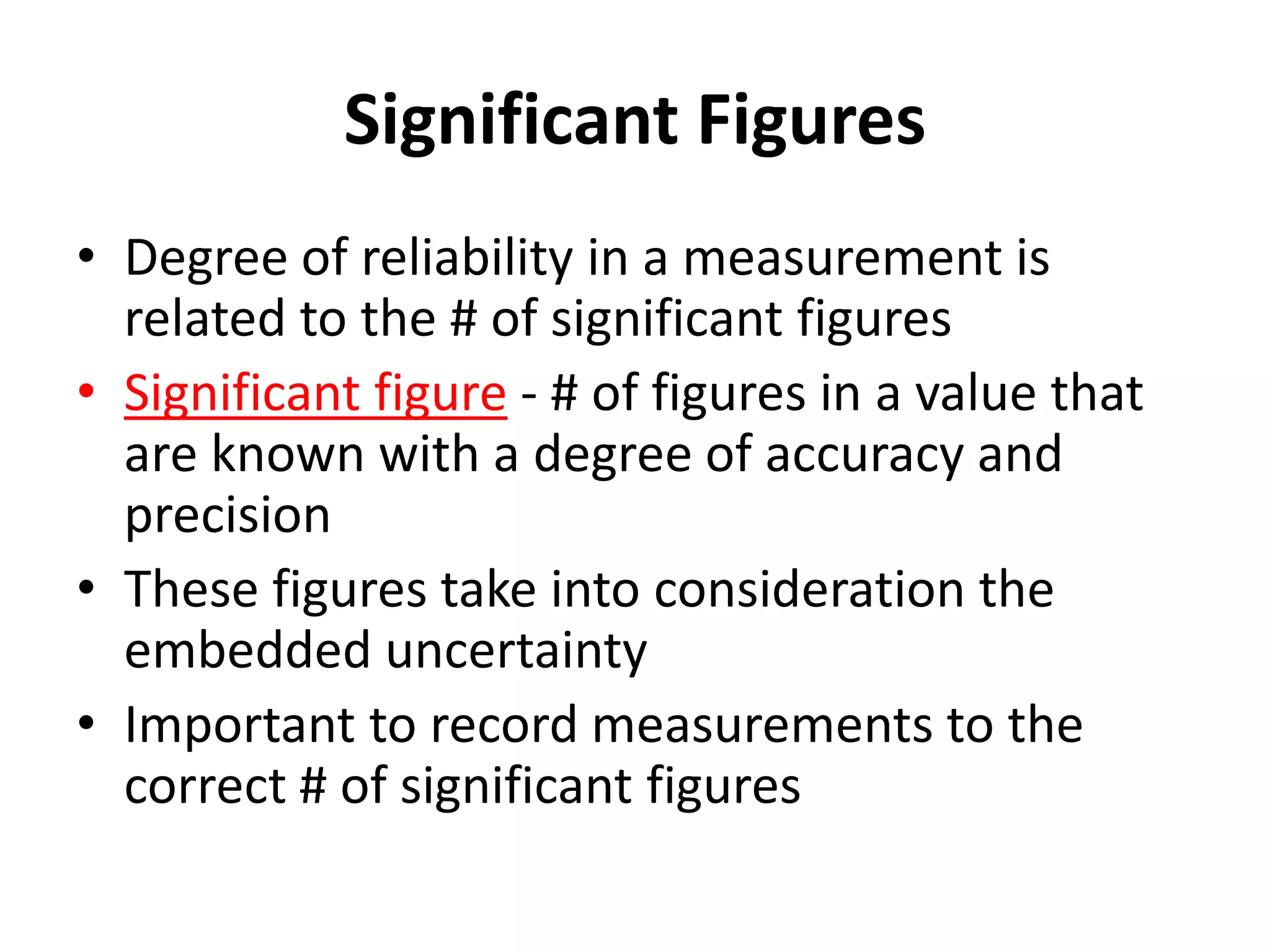
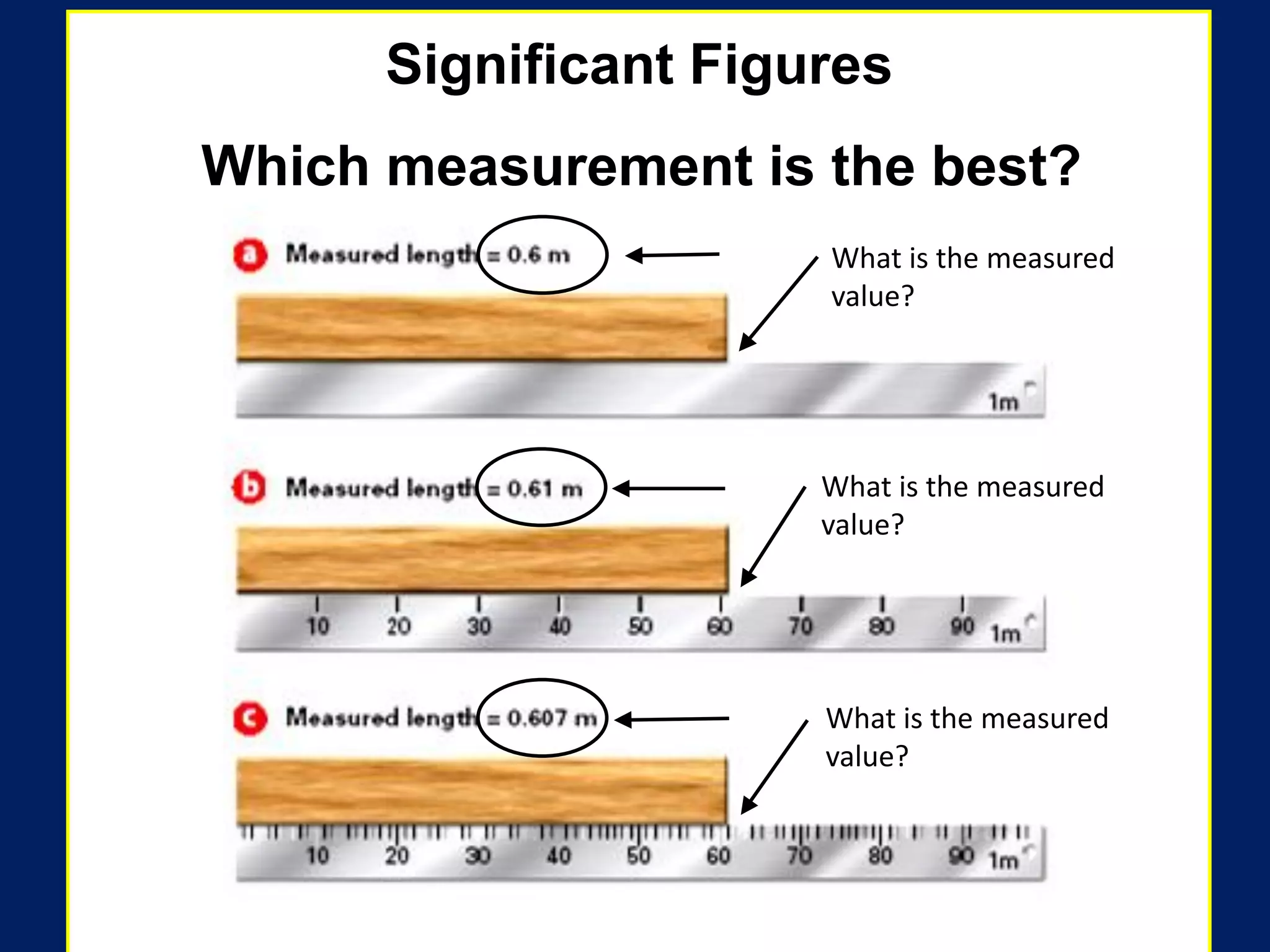
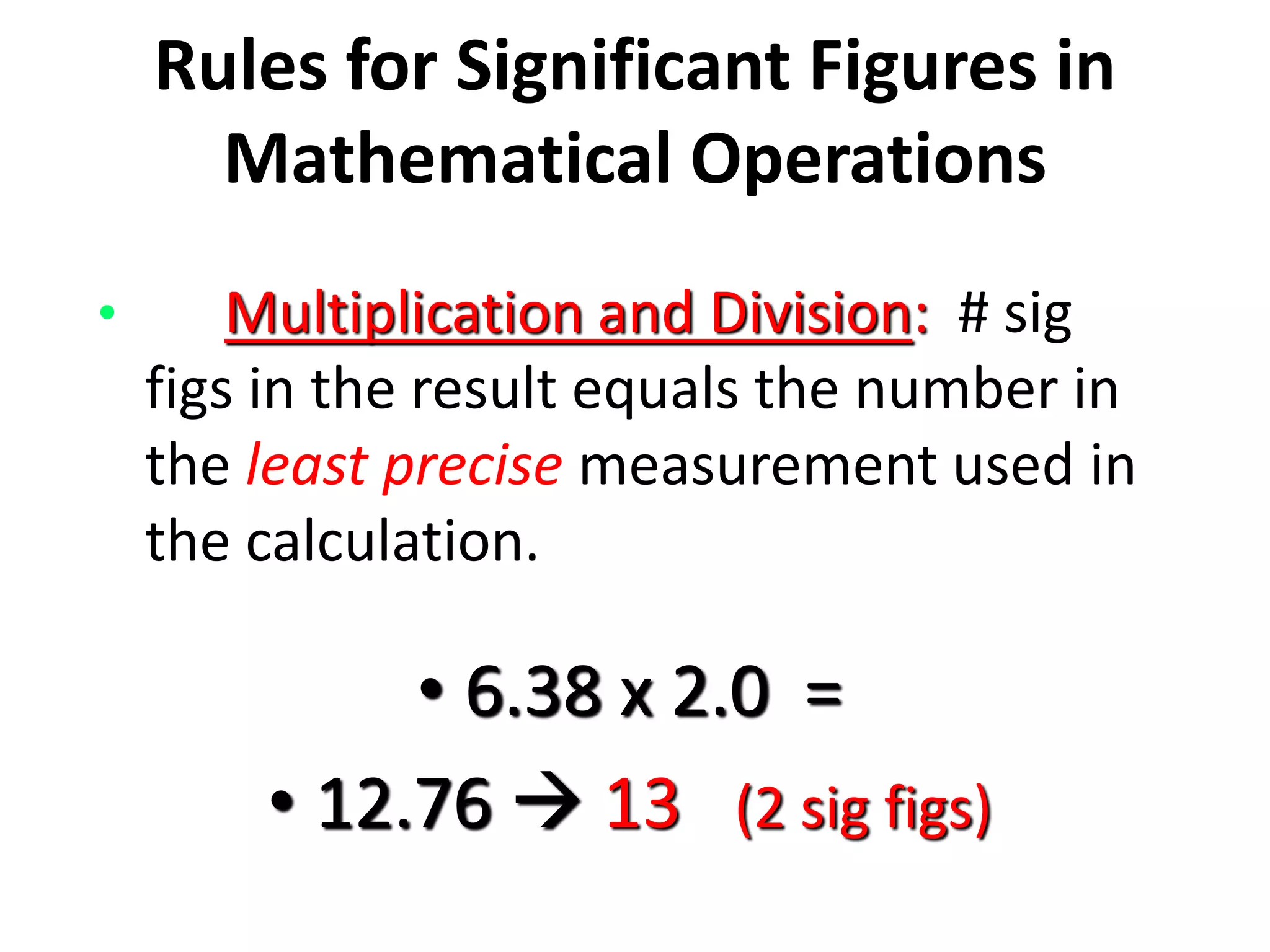
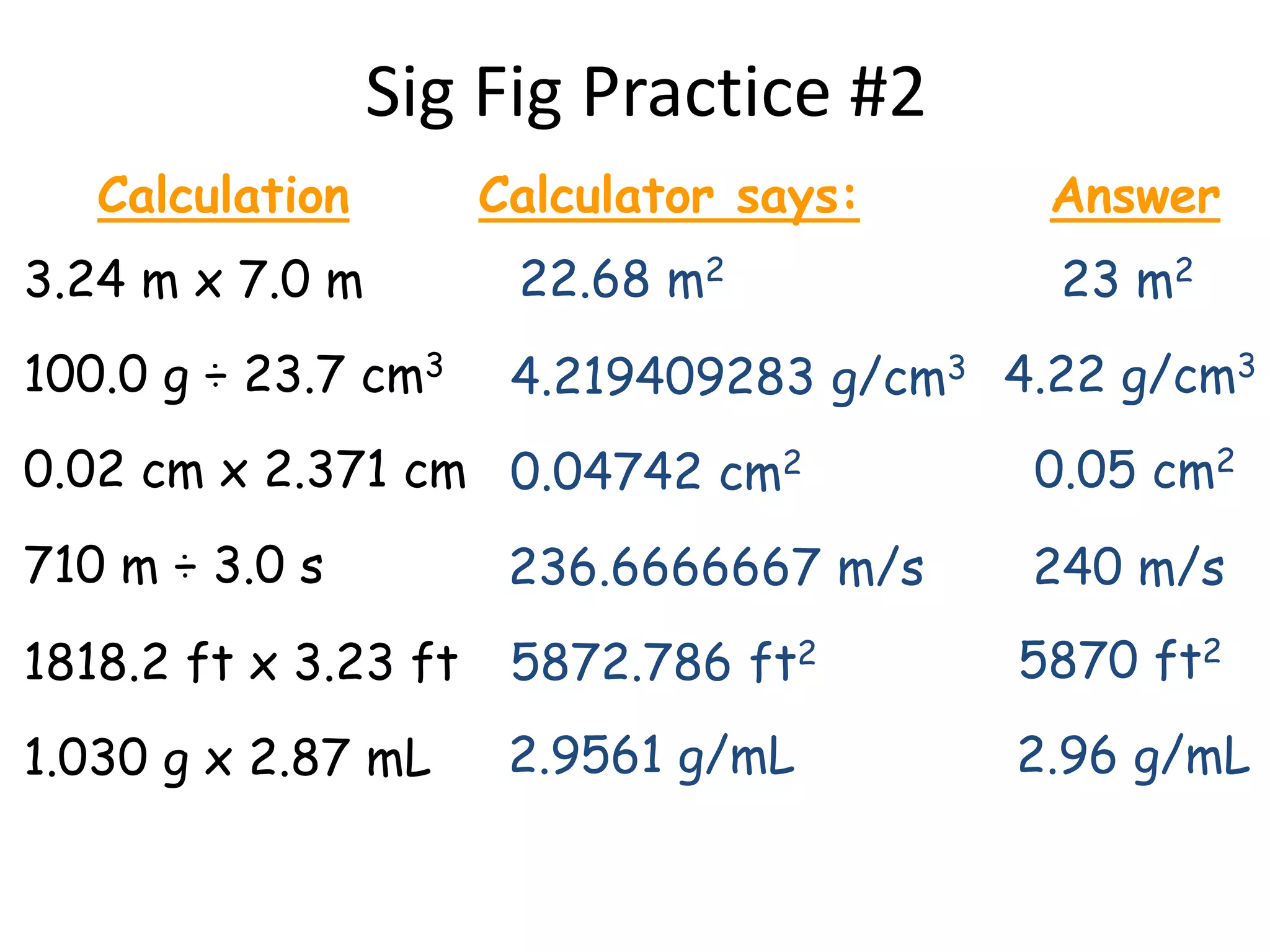
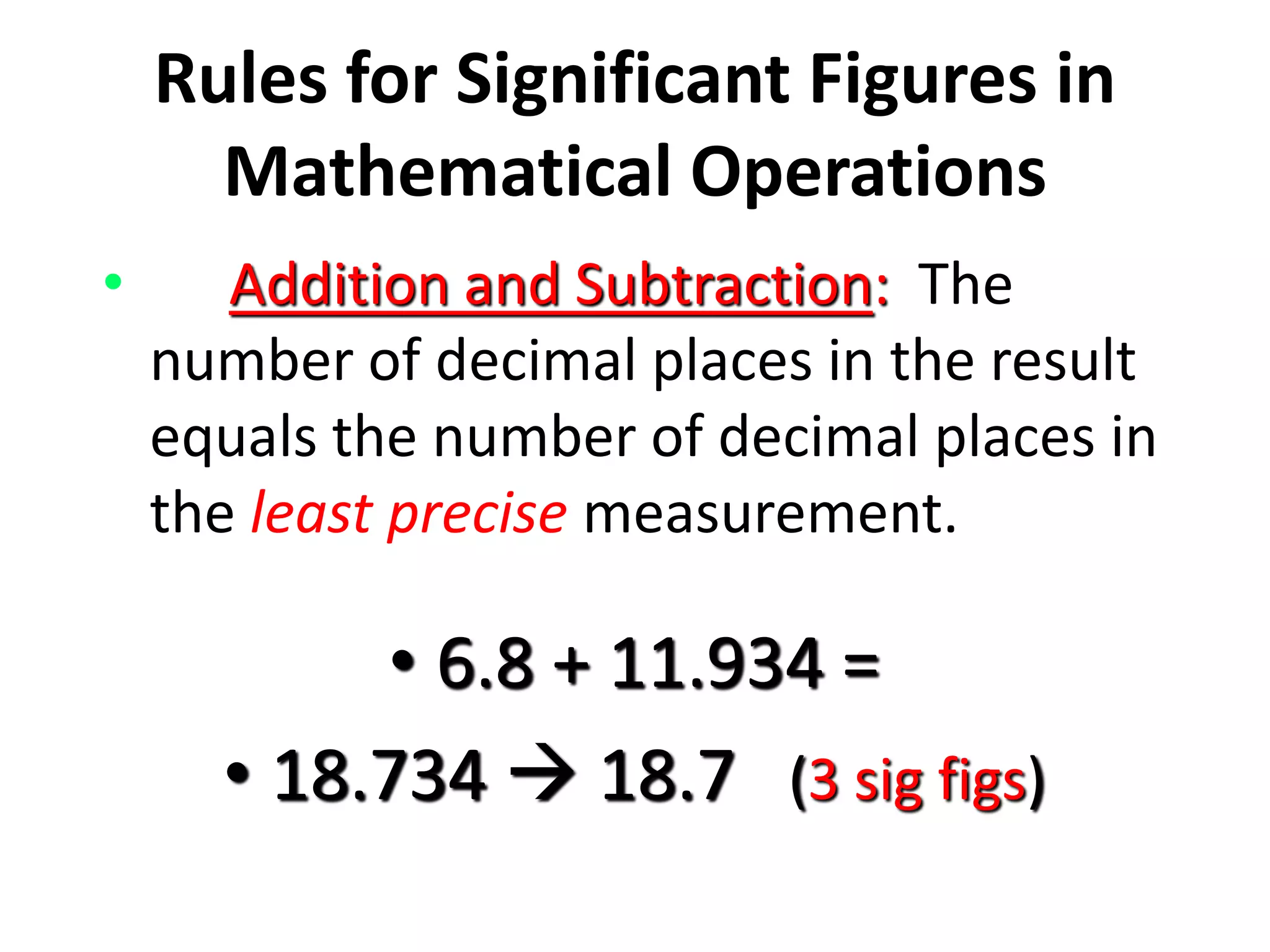
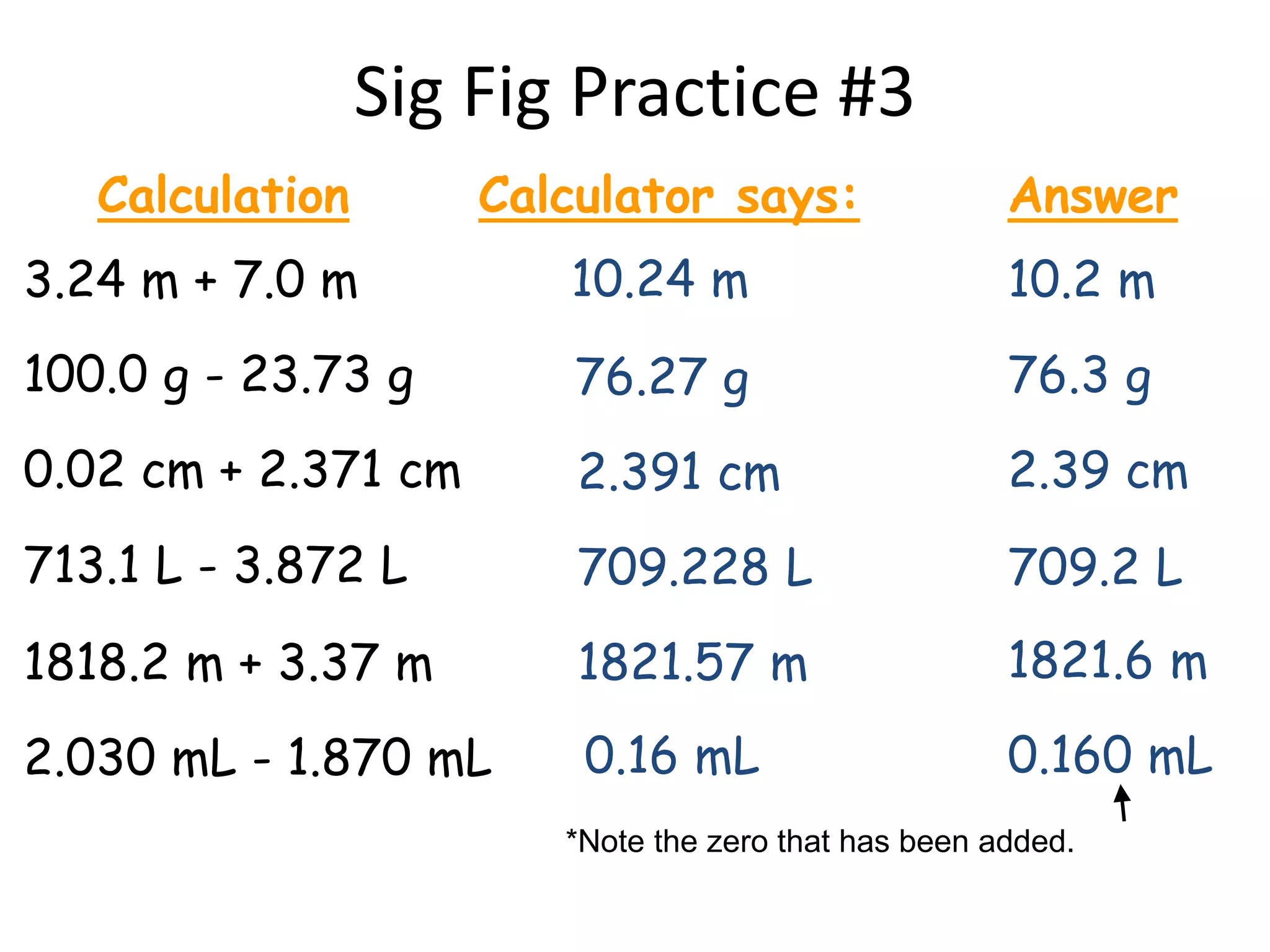
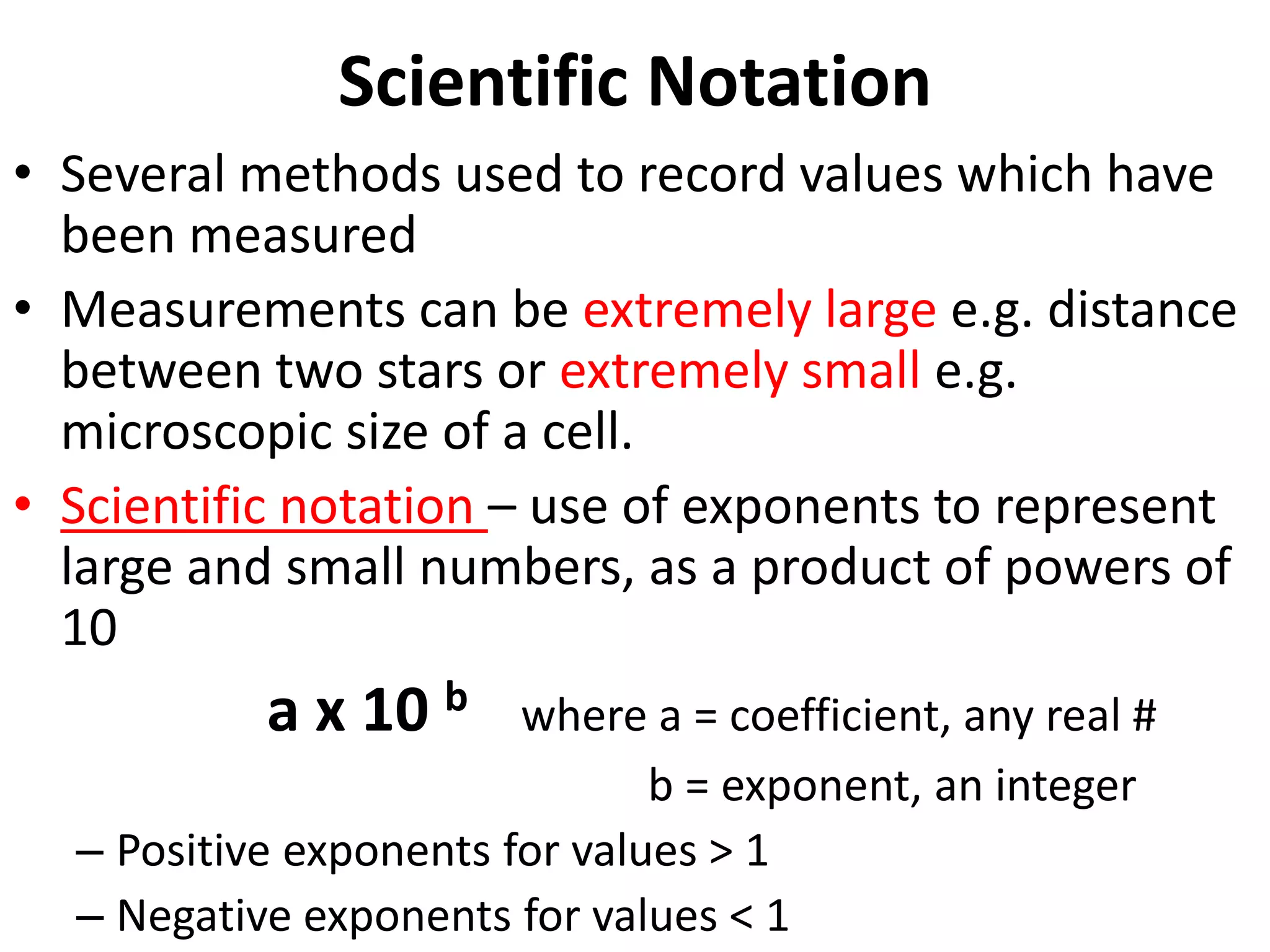
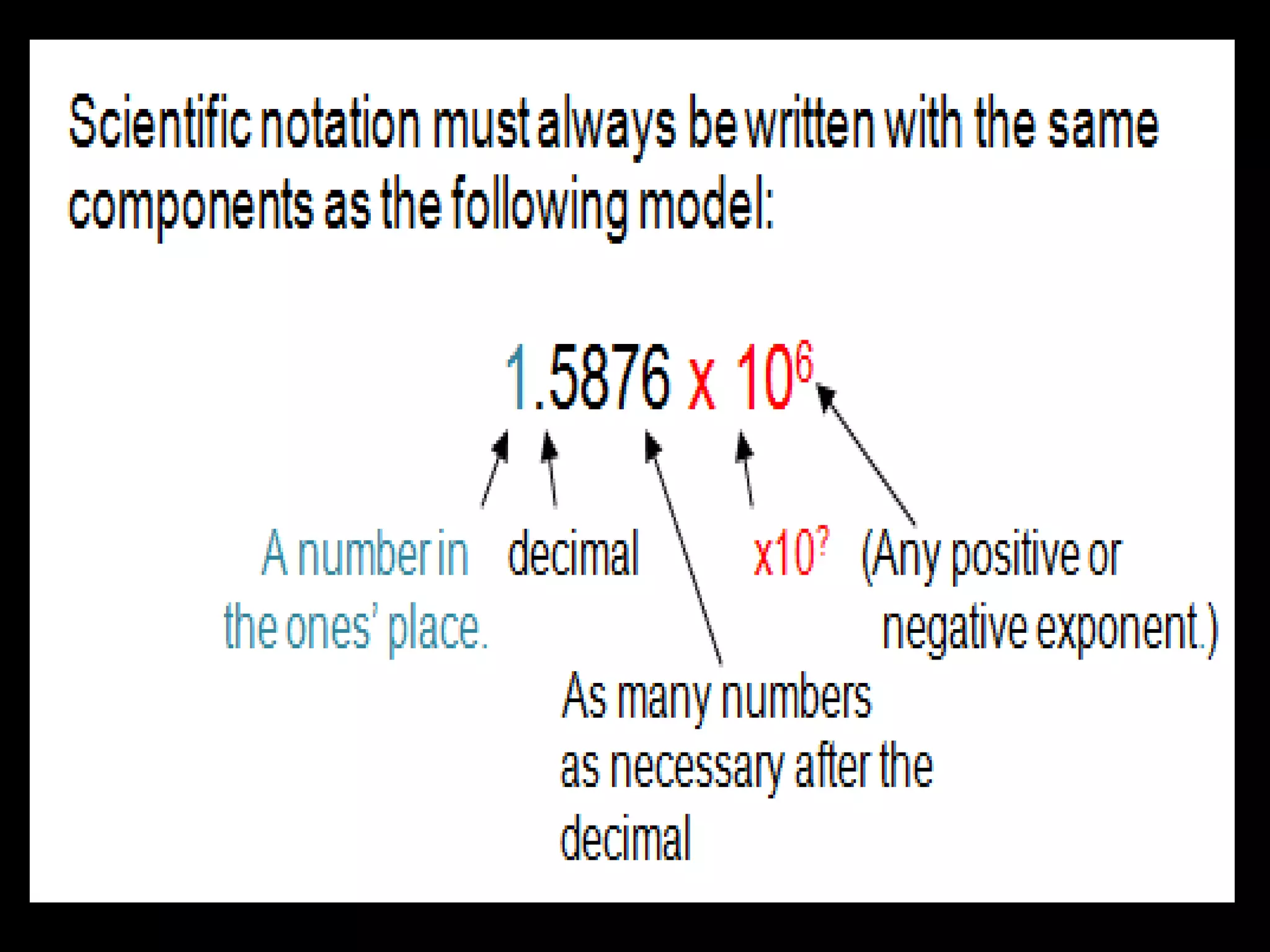
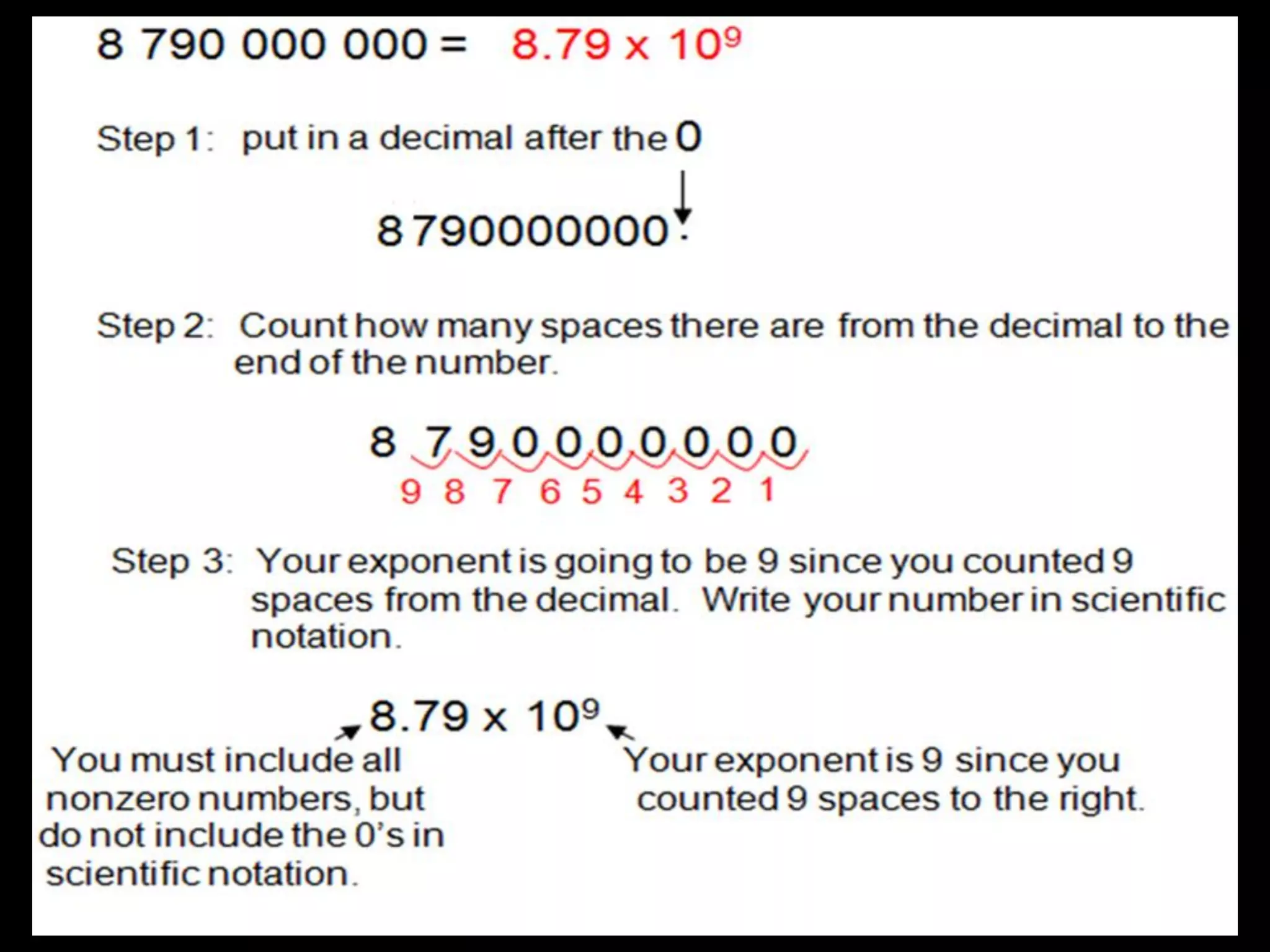
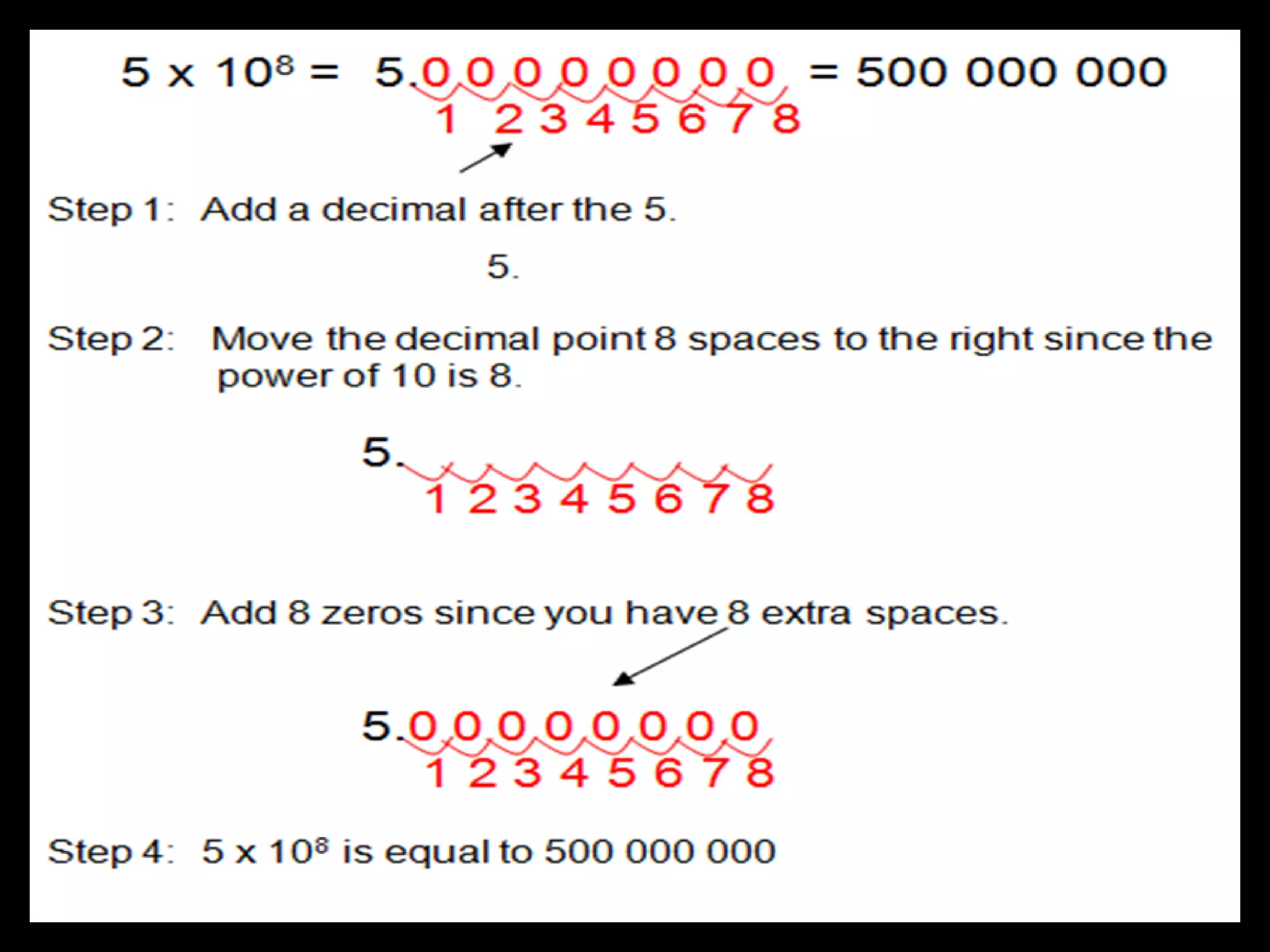
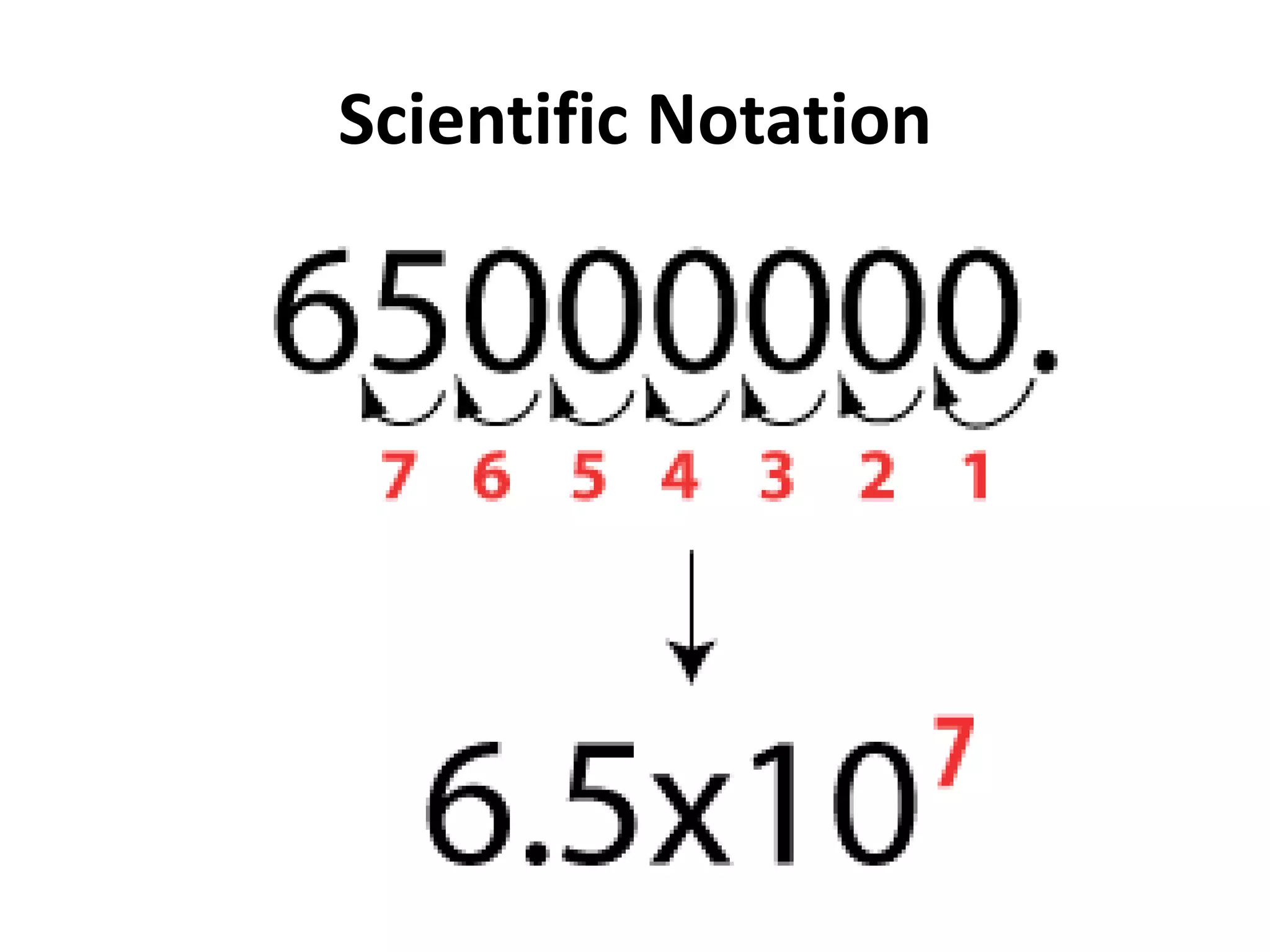
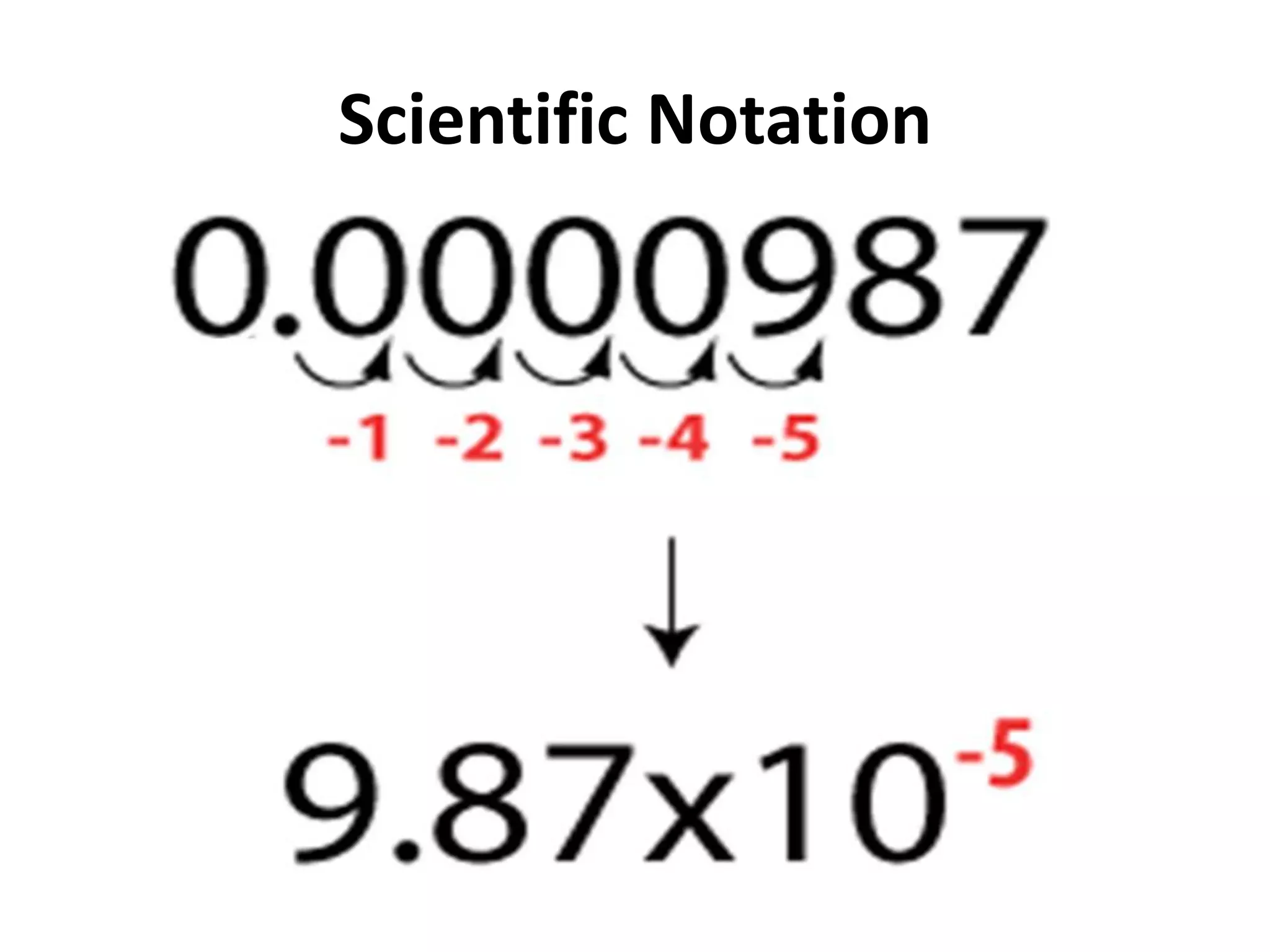
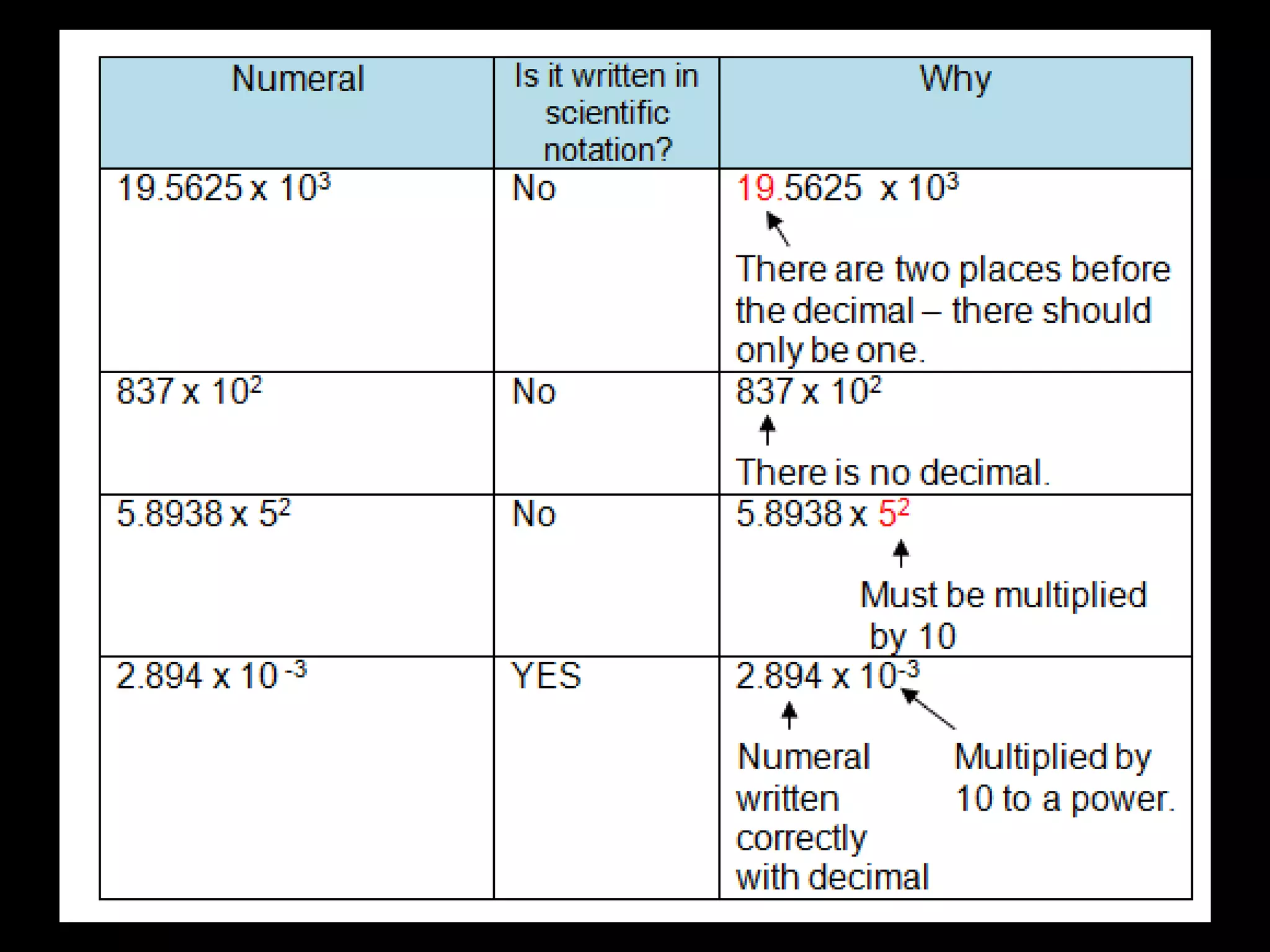
4. It covers converting measurements to scientific notation and the importance of significant figures.