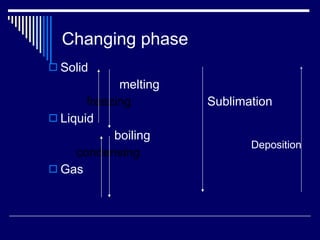
The document introduces chemistry as the study of matter. Matter is anything that has mass and volume. Mass refers to the amount of matter in an object, while volume refers to the amount of space an object occupies. Atoms are the basic units that make up matter and molecules form when two or more atoms are bonded. Matter can be classified as pure substances or mixtures. Pure substances are either elements or compounds, while mixtures are either homogeneous or heterogeneous. The three common states of matter are solids, liquids, and gases.