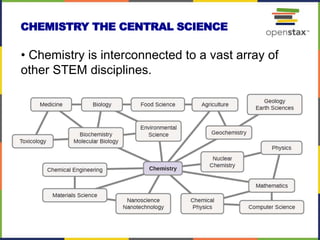
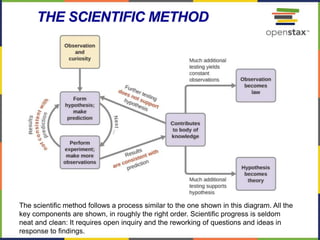
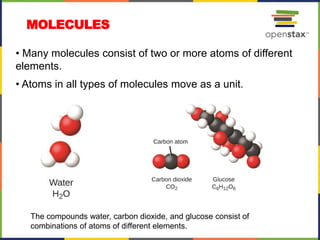
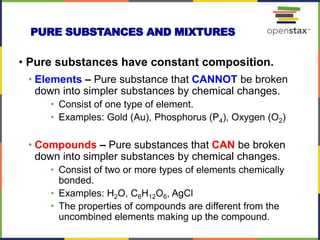
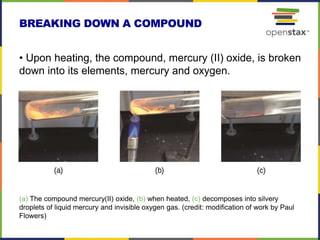
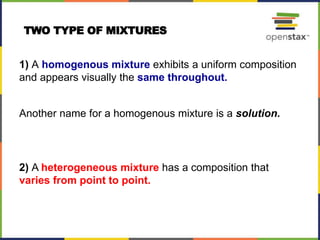
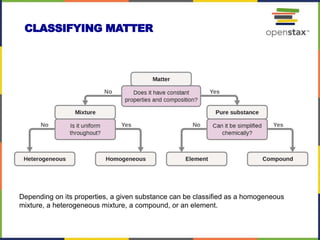
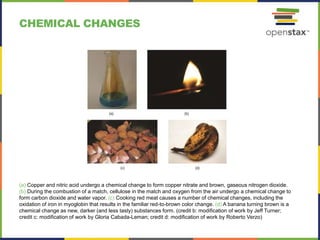
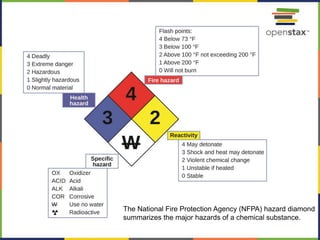
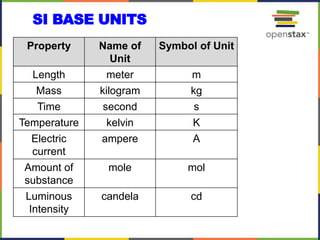
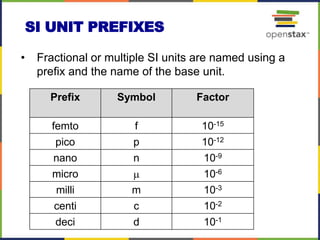
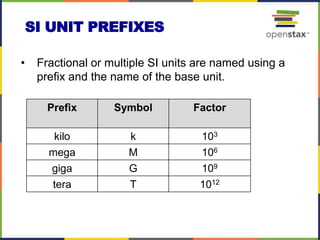
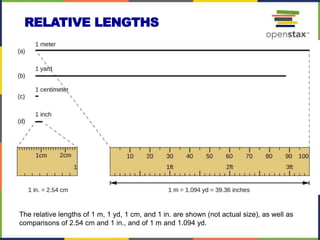
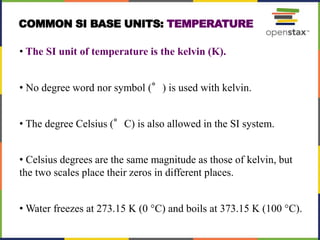
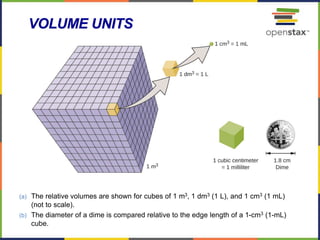
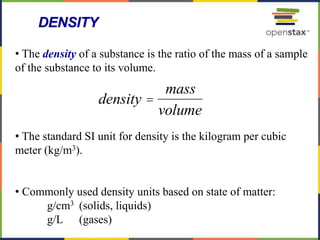
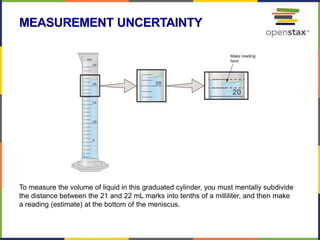
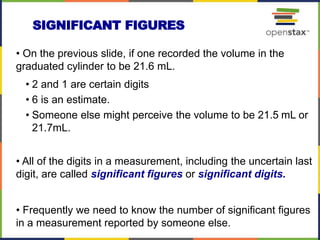
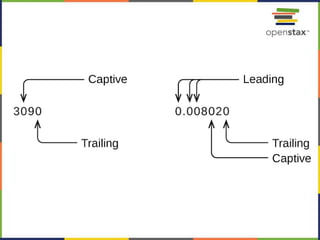
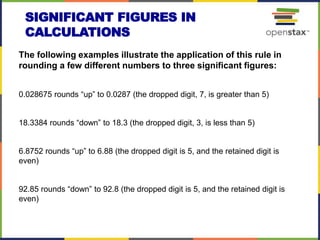
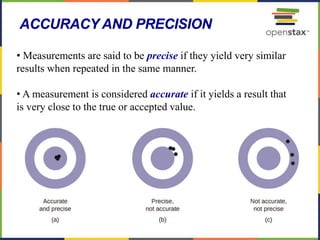
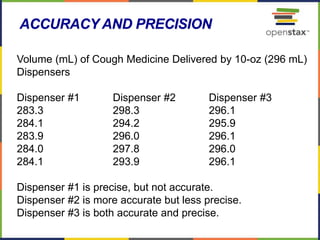
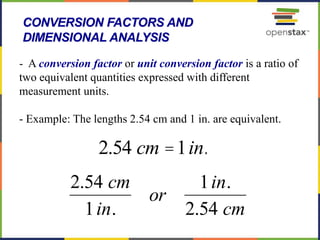
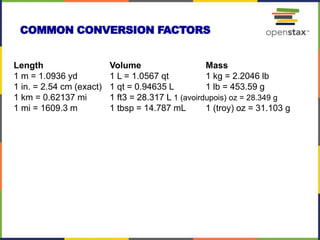
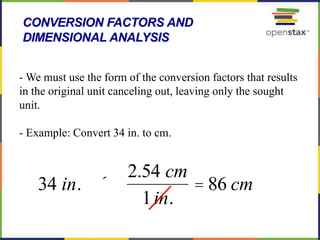
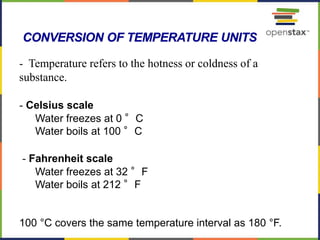
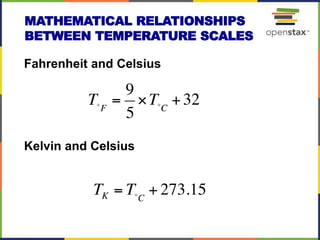
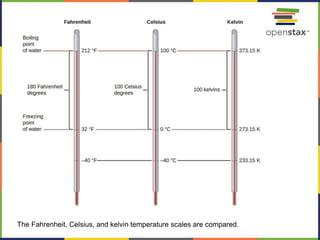
This document provides an overview of key concepts in chemistry. It discusses the study of matter and its composition, properties, and interactions. Matter can exist in various phases and be classified as elements, compounds, or mixtures. Properties include physical characteristics that do not change composition and chemical properties that involve compositional changes. Measurements in chemistry require units and have uncertainty. The document outlines common units in the International System of units and concepts like accuracy and precision in reported values.