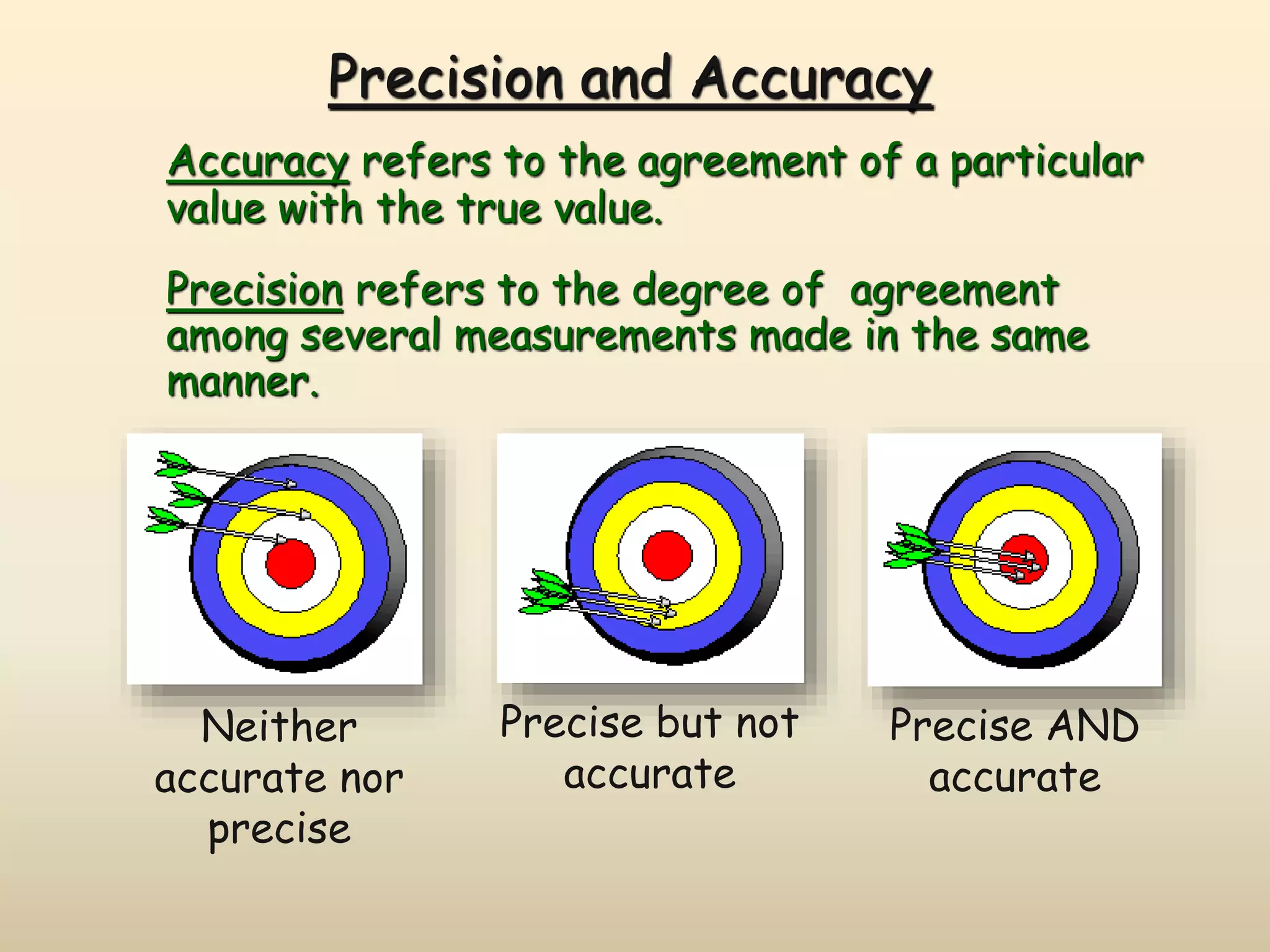
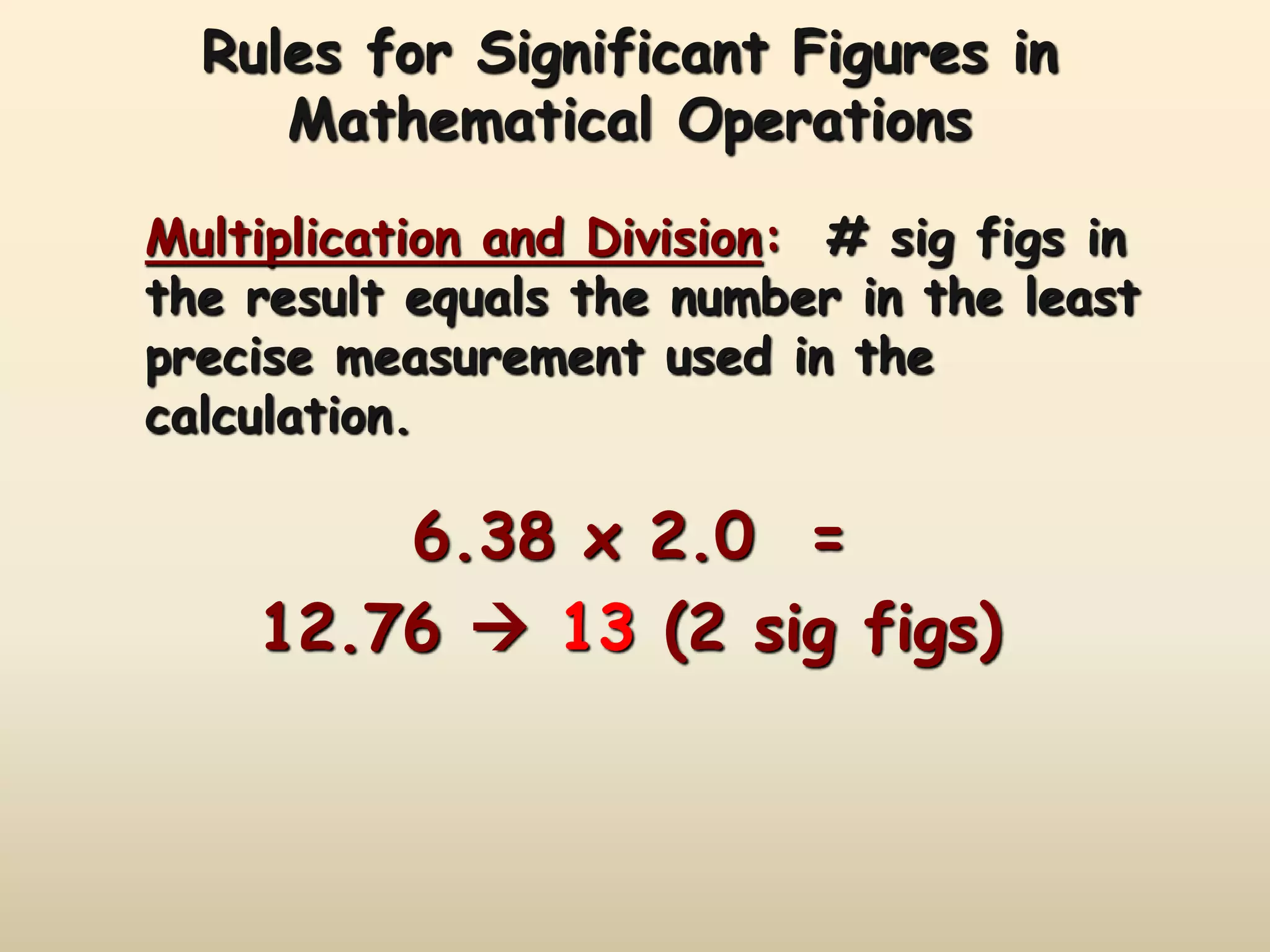
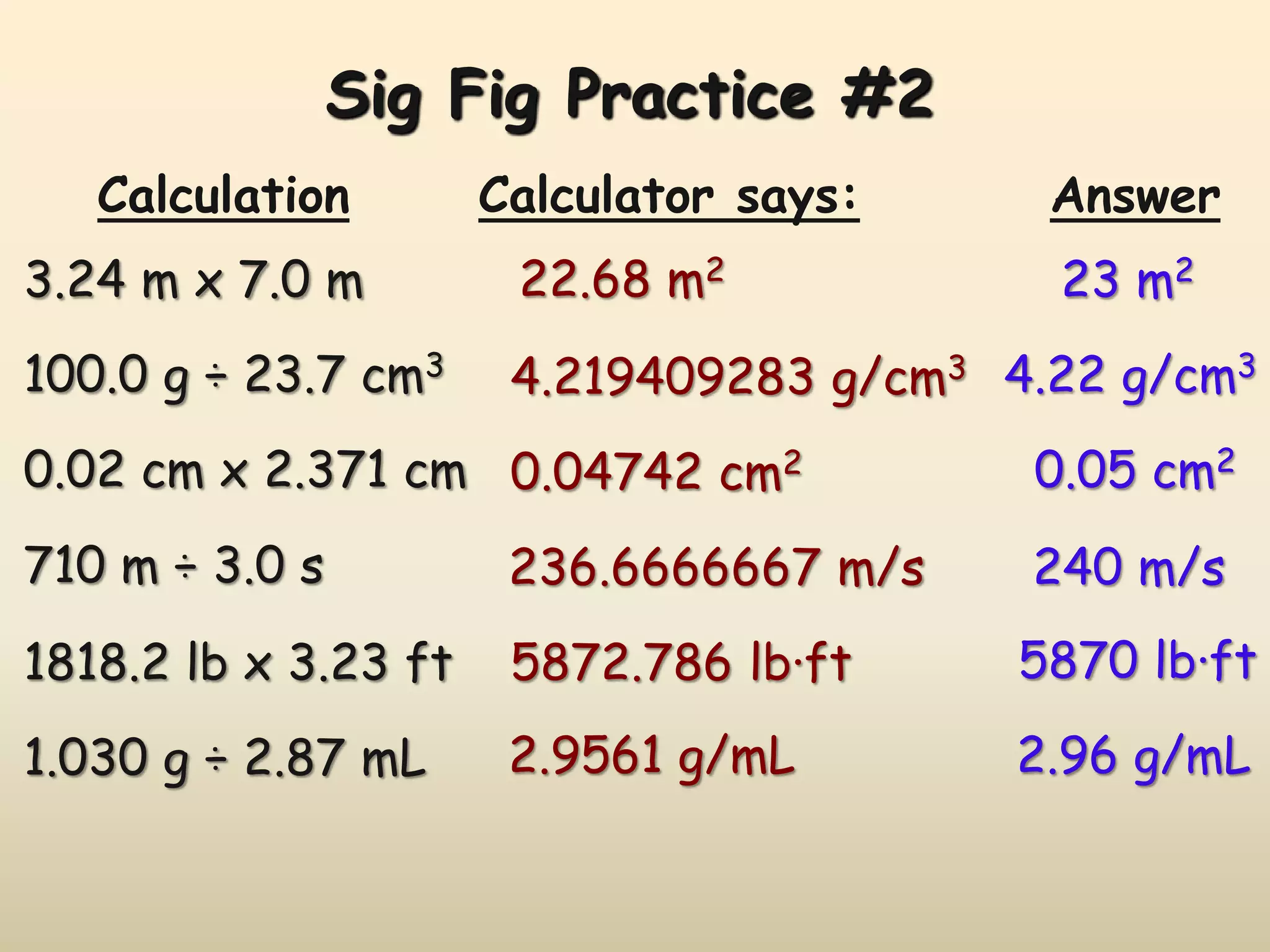
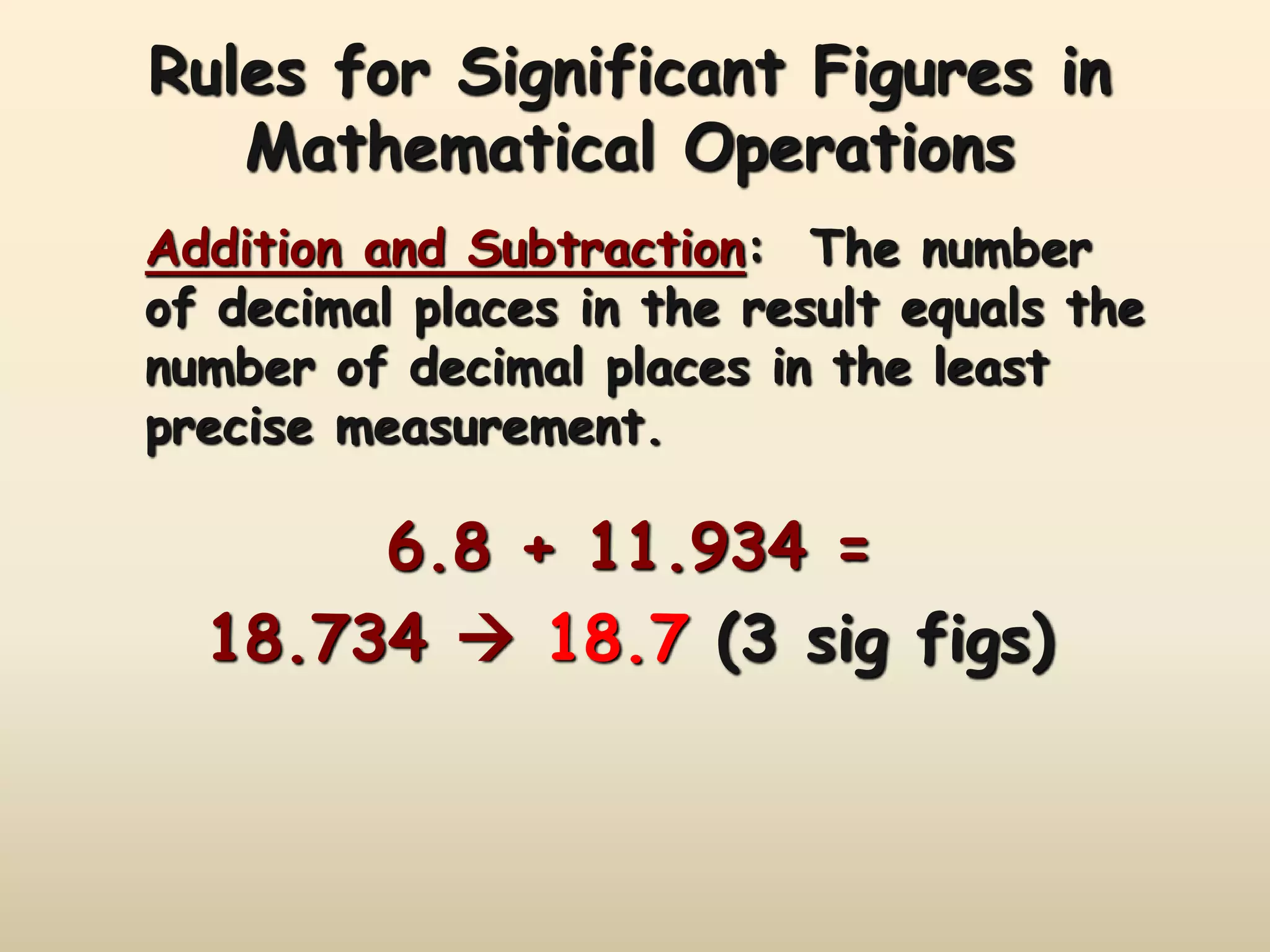
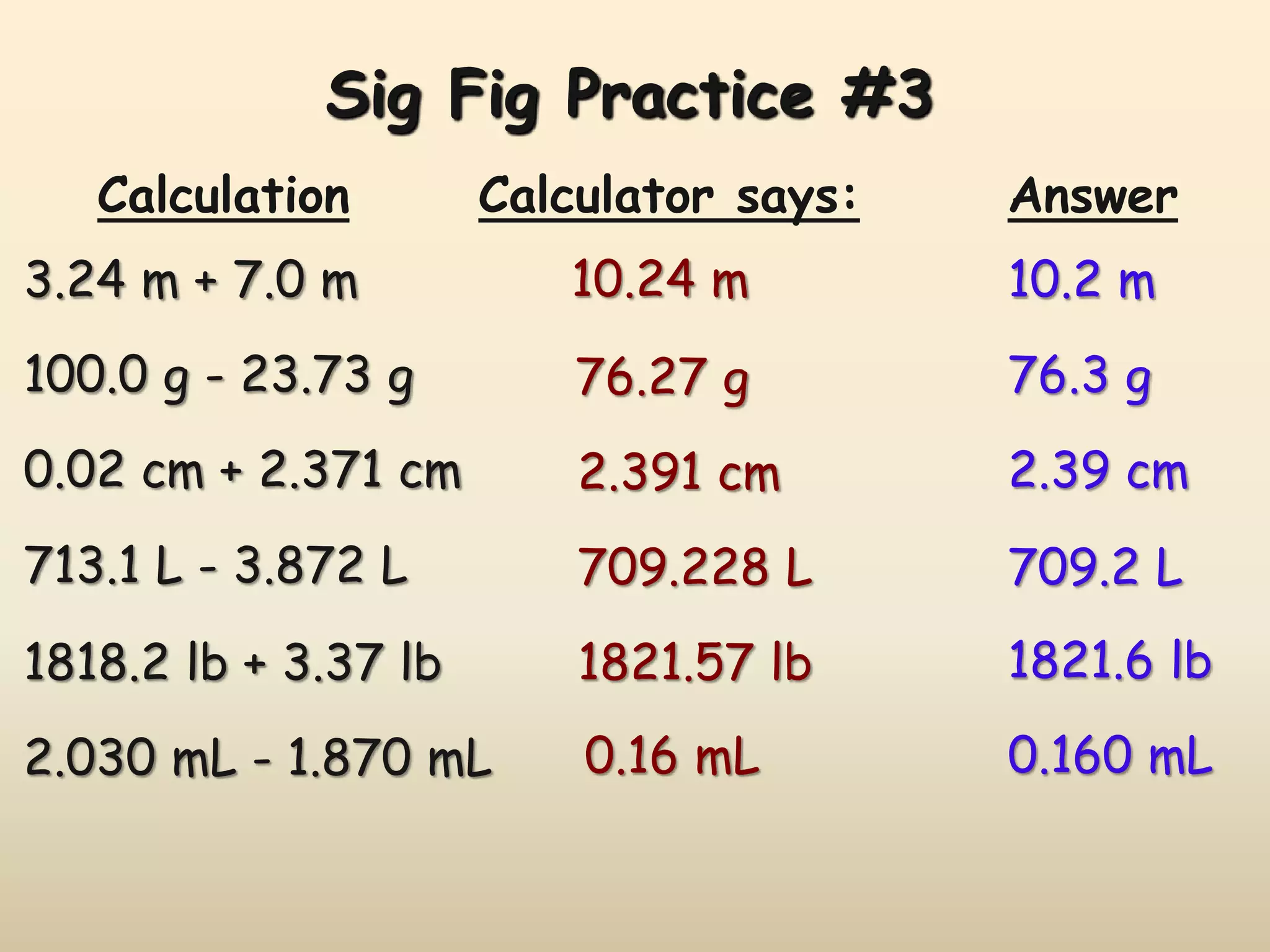
This document discusses uncertainty in measurement and significant figures. It explains that measurements have uncertainty due to limitations of instruments. Precision refers to the agreement between repeated measurements while accuracy is the agreement with the true value. There are two types of errors - random errors that can be high or low, and systematic errors that are always in the same direction. The document provides rules for determining the number of significant figures in measurements and calculations, including how significant figures are treated in addition, subtraction, multiplication and division.