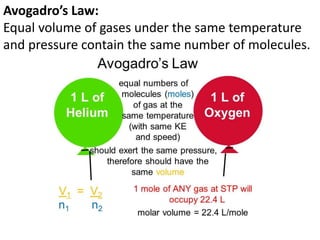
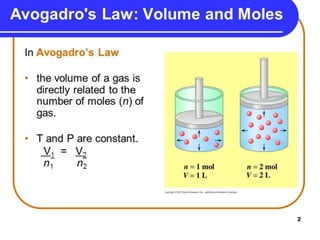
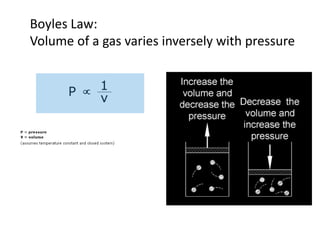
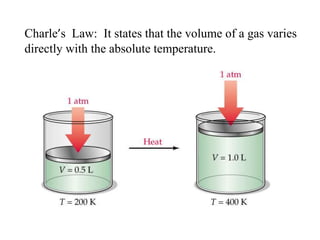
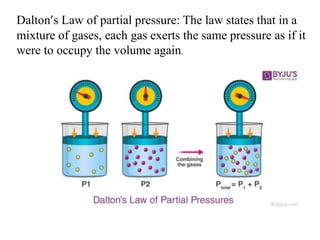
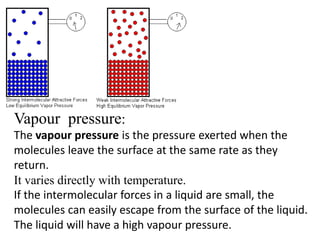
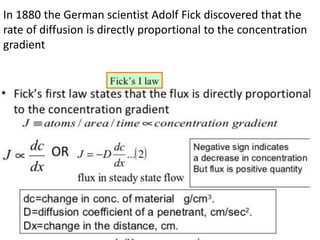
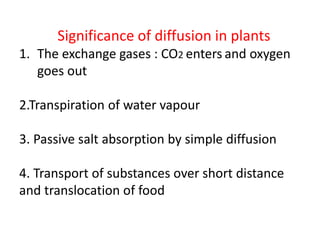
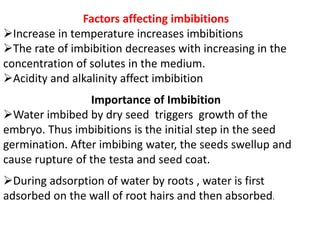
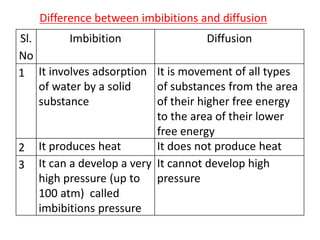
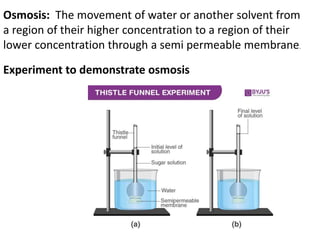
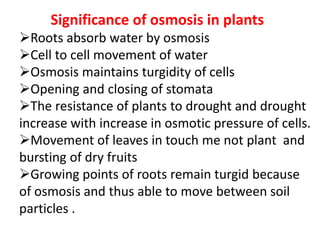
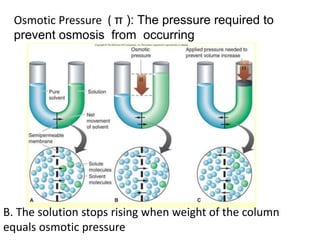
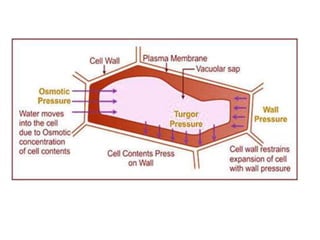
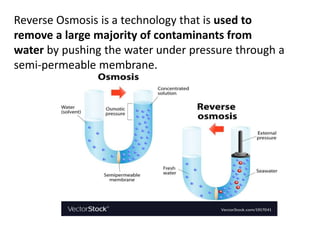
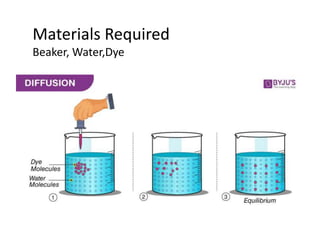
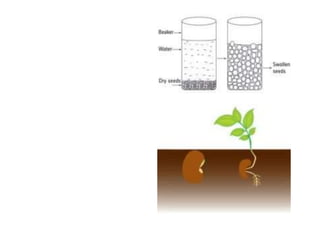
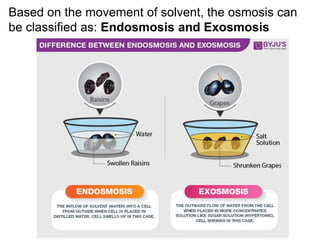
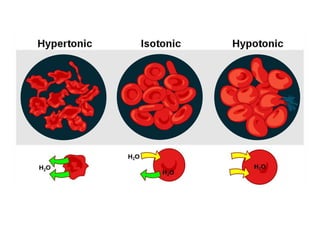
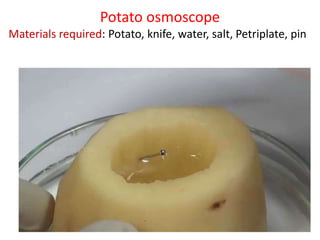
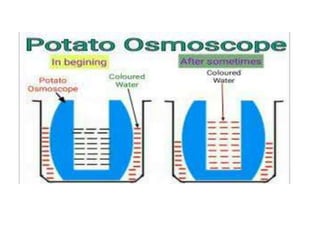
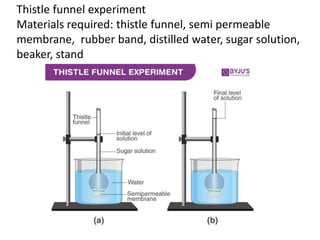
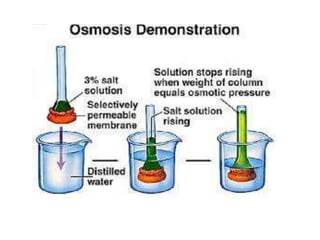
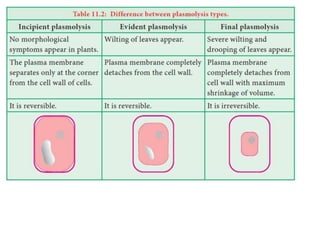
The document discusses various concepts related to diffusion, imbibition, and osmosis. It defines key terms like Boyle's law, Charles' law, Dalton's law of partial pressure, Henry's law, Graham's law, vapour pressure, diffusion, facilitated diffusion, simple diffusion, imbibition, endosmosis, exosmosis, hypotonic solution, hypertonic solution, isotonic solution, osmotic pressure, turgor pressure, and wall pressure. It also discusses the significance and practical demonstration of these concepts, as well as reverse osmosis.