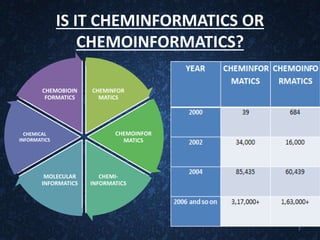
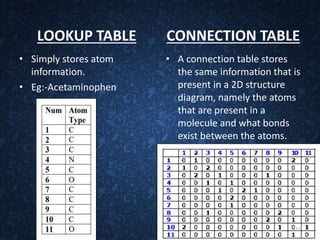
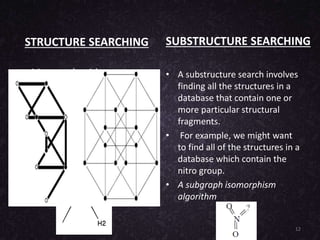
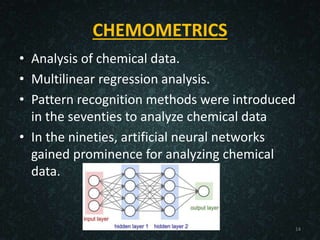
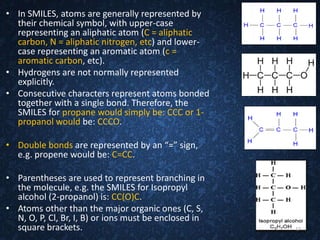
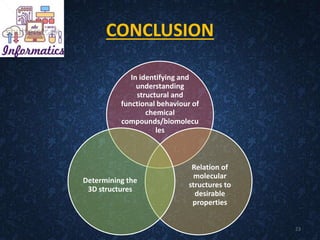
The document provides an overview of cheminformatics, detailing its definition, importance, and applications in chemistry and drug discovery. It emphasizes the organization and analysis of chemical data, the tools used, and the significance of cheminformatics as a distinct discipline. Additionally, it touches upon aspects like molecular modeling, data management, and the role of cheminformatics in predictive analysis across various chemistry fields.