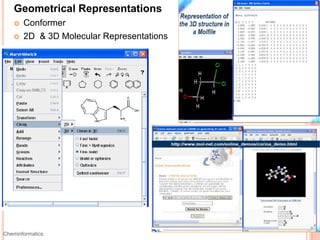
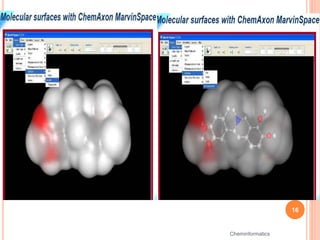
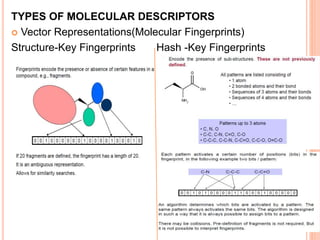
The document provides an overview of cheminformatics, detailing its definition, historical evolution, and various applications in drug discovery and molecular design. It highlights key concepts such as molecular descriptors, cheminformatics tasks, and high-throughput screening, along with the evolution of significant chemical databases. The paper emphasizes the importance of cheminformatics in modern chemical research, illustrating how it aids in the analysis and design of chemical compounds.