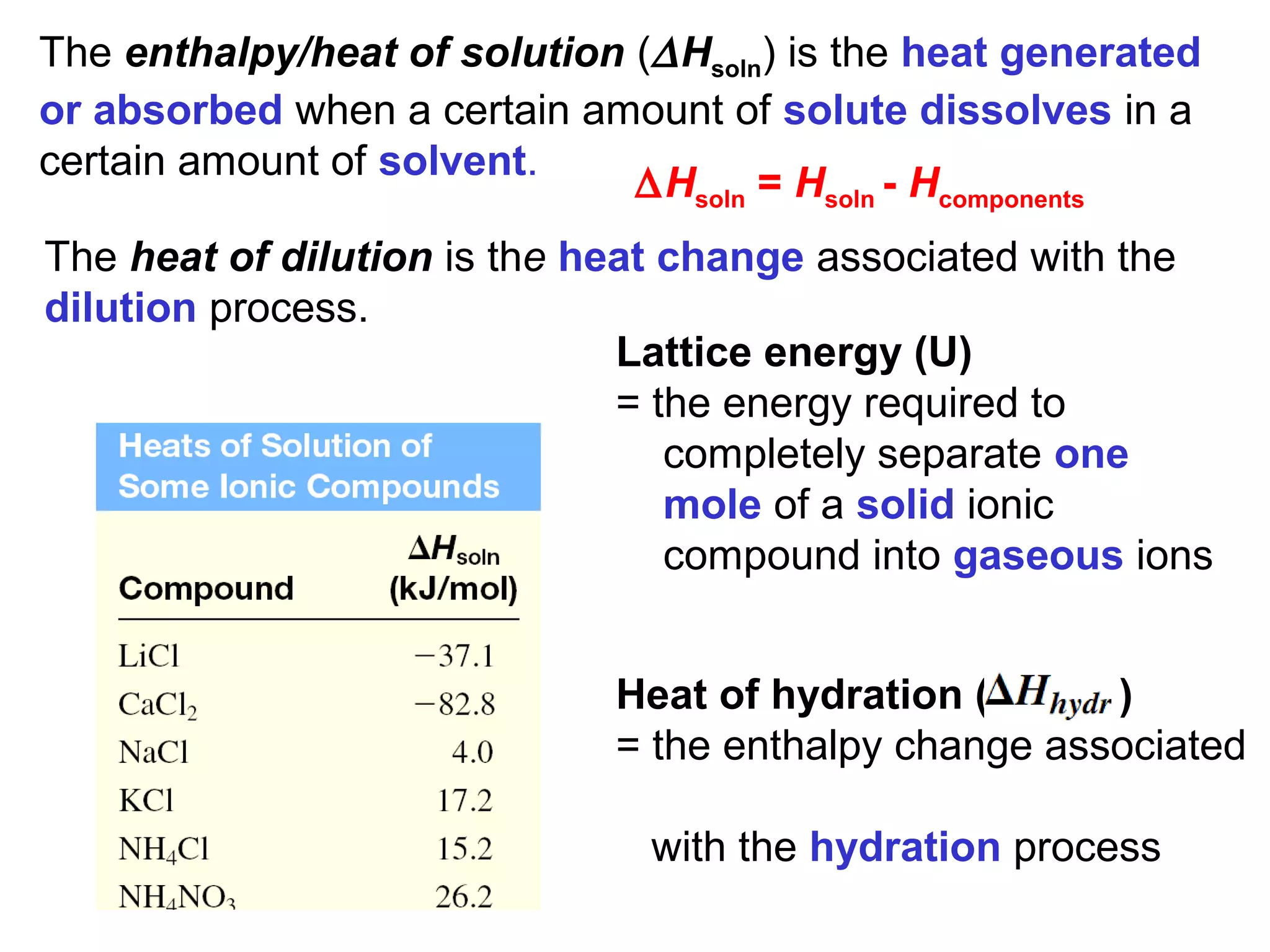
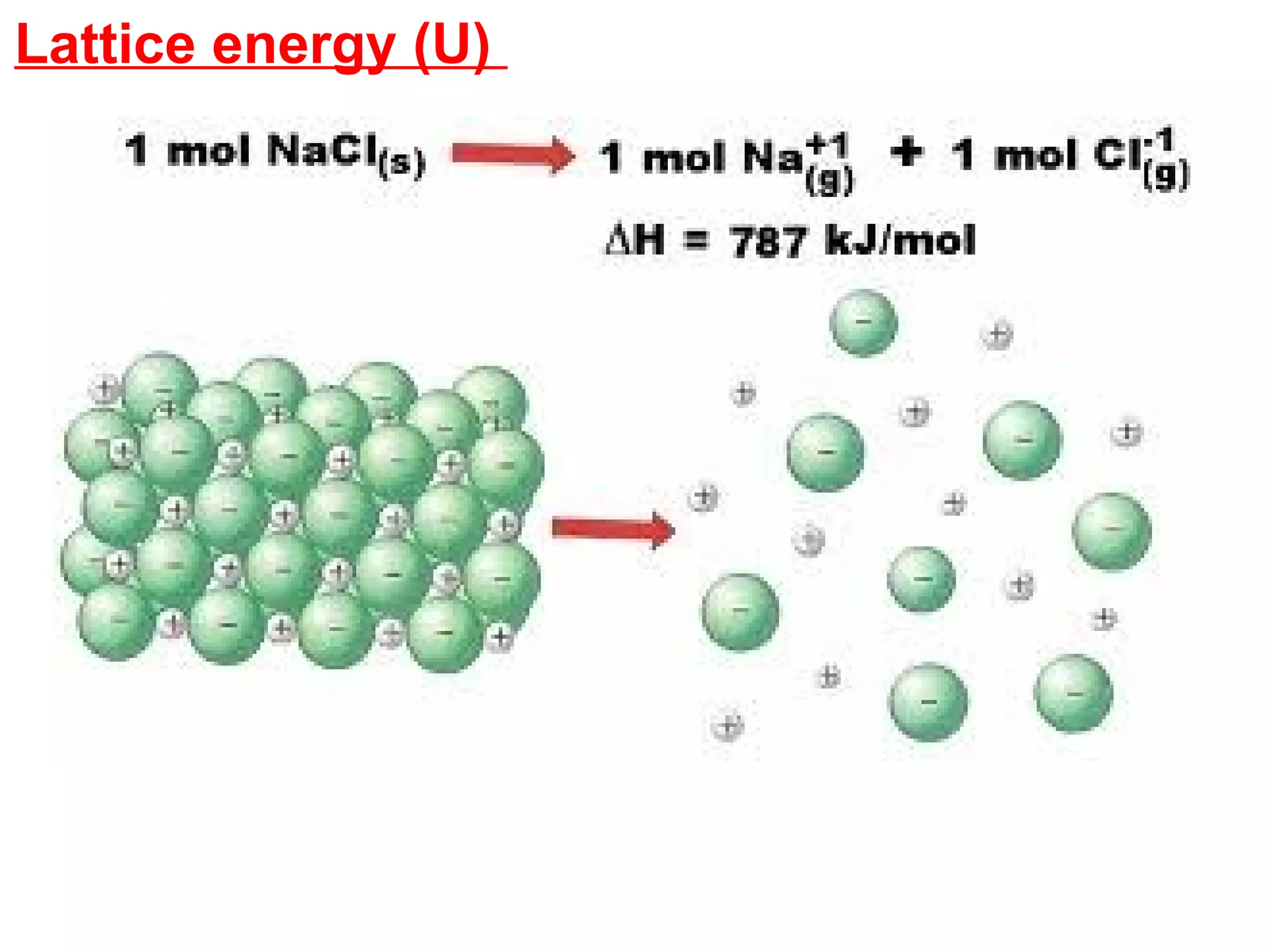
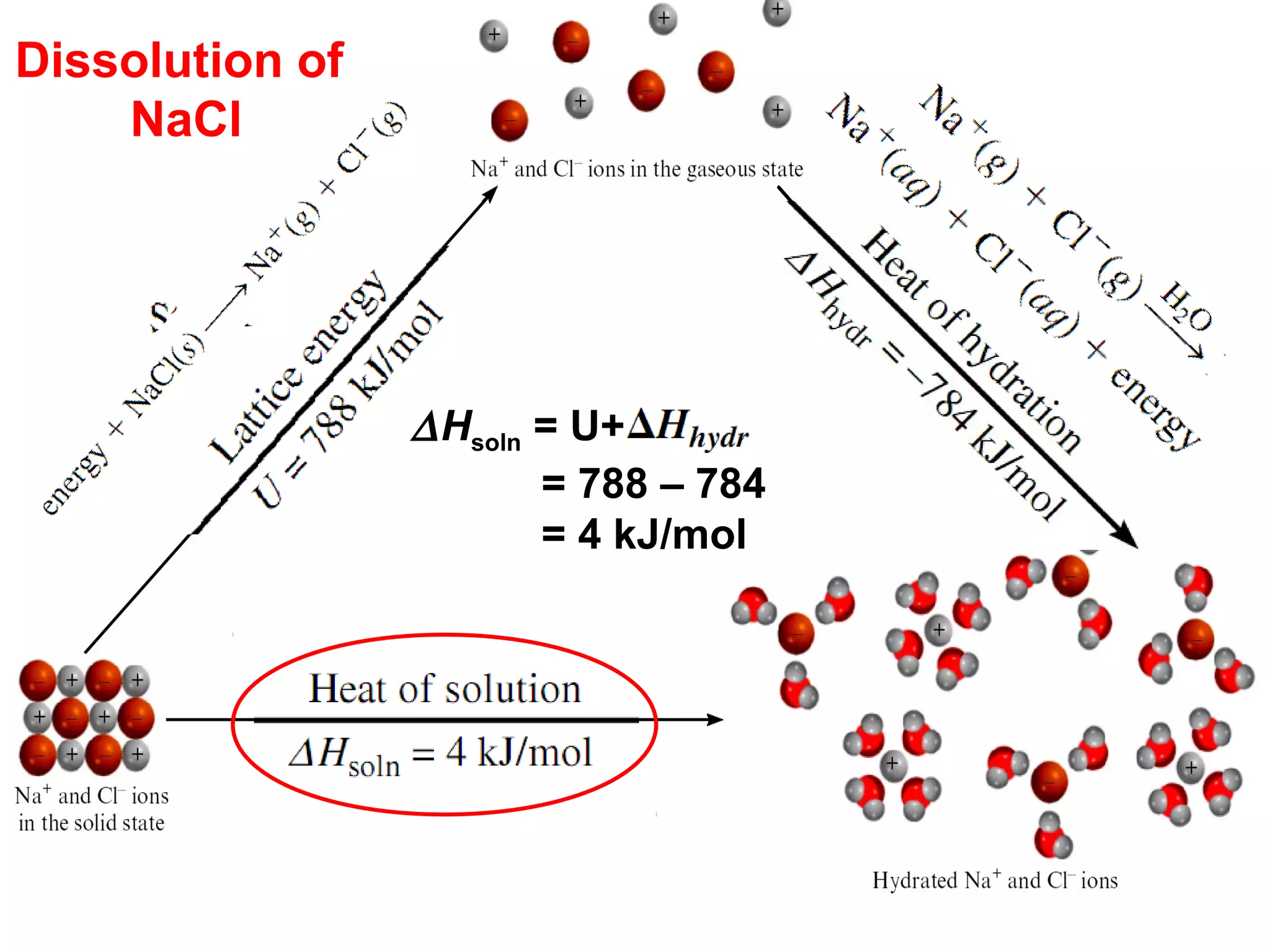
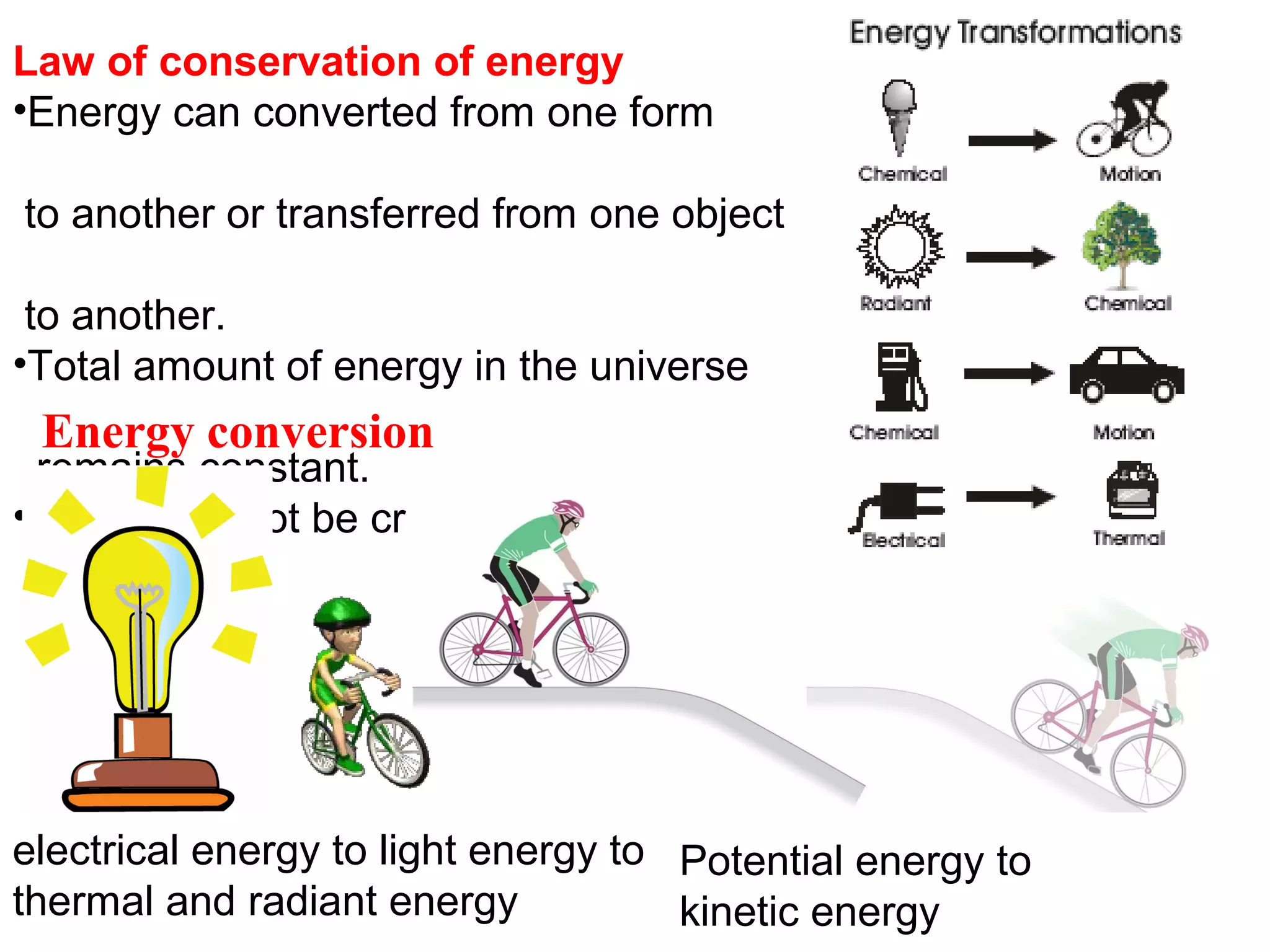
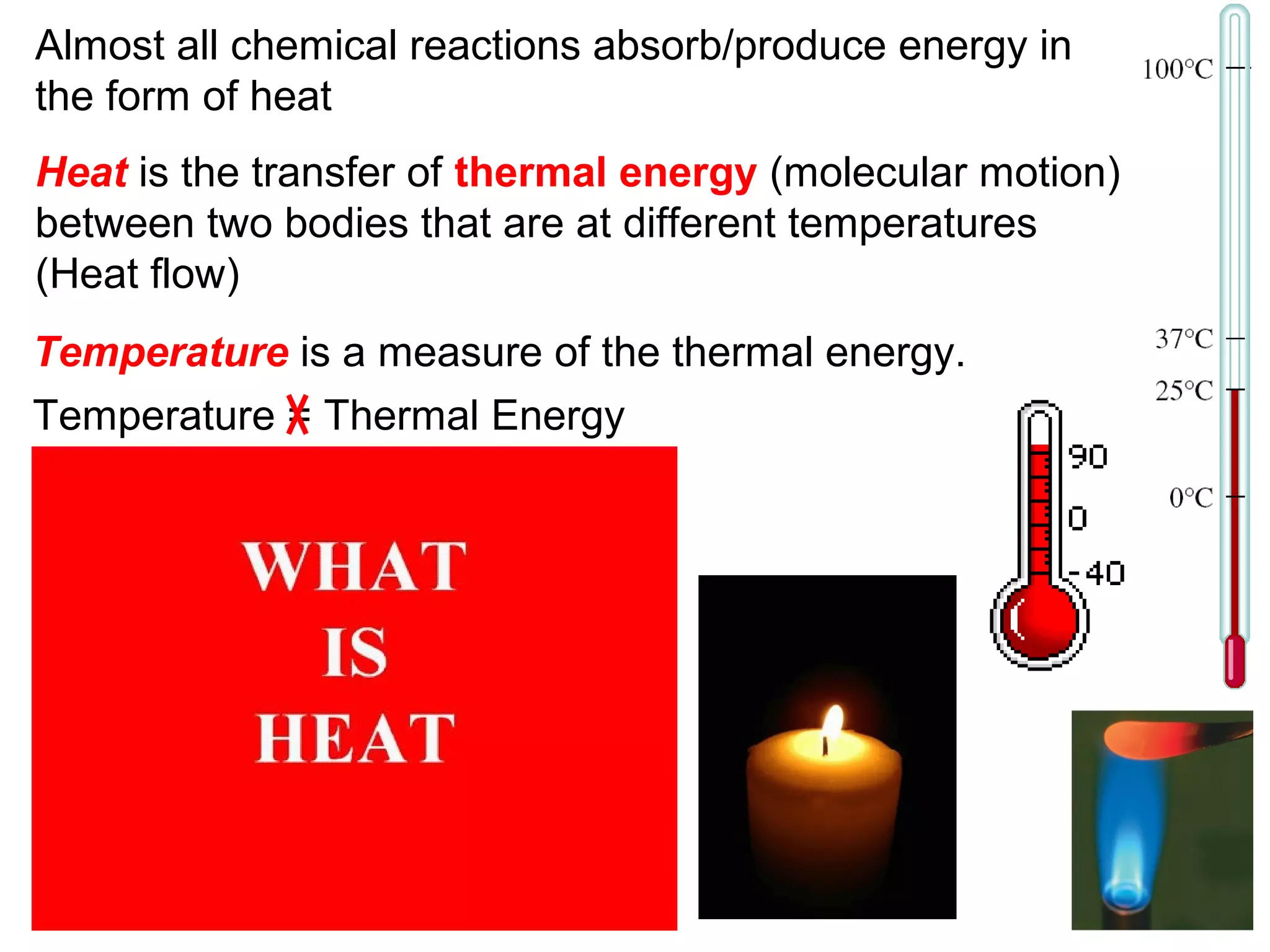
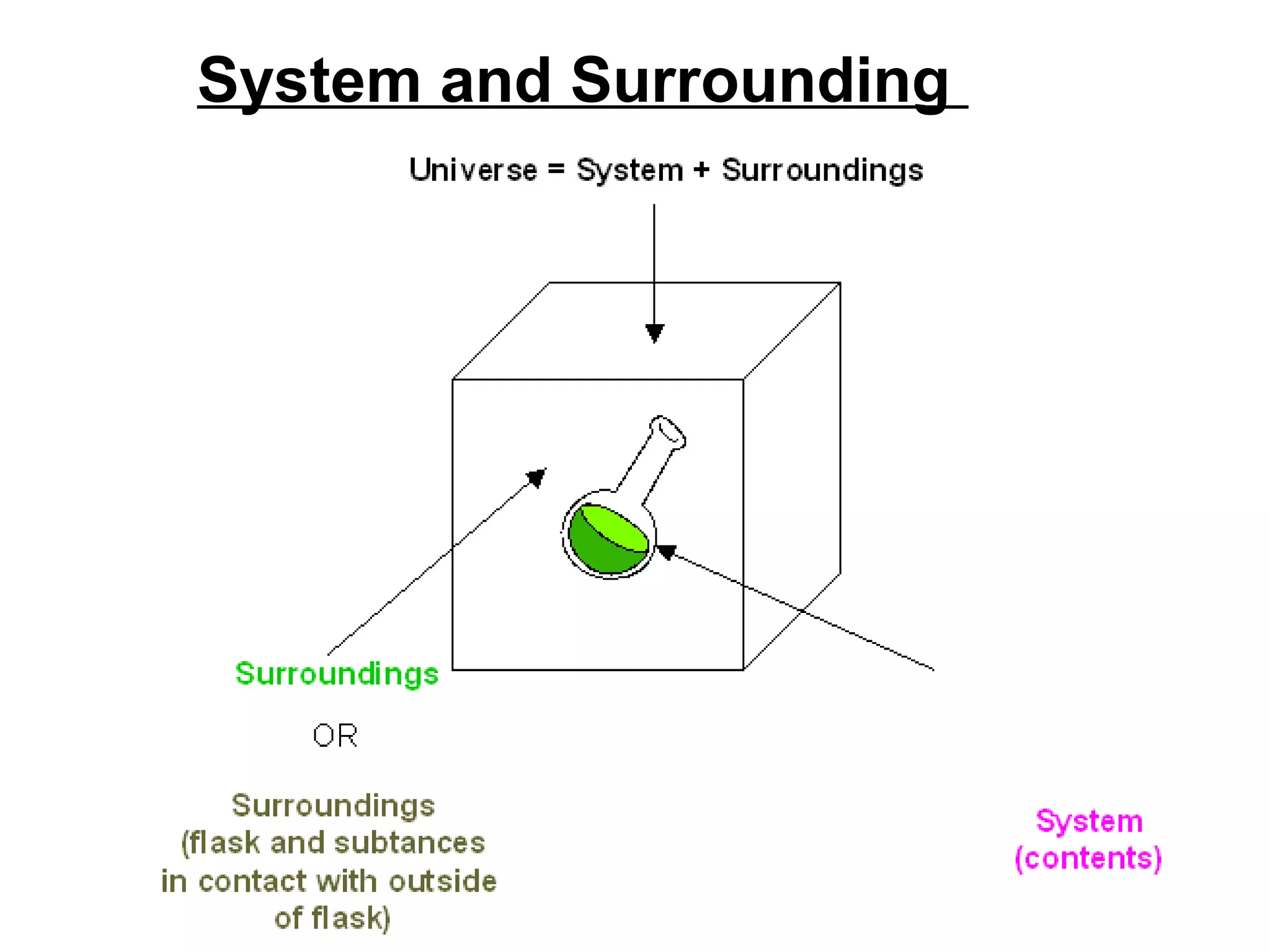
The document discusses different types of energy and energy changes that occur during chemical reactions. It defines key concepts such as:
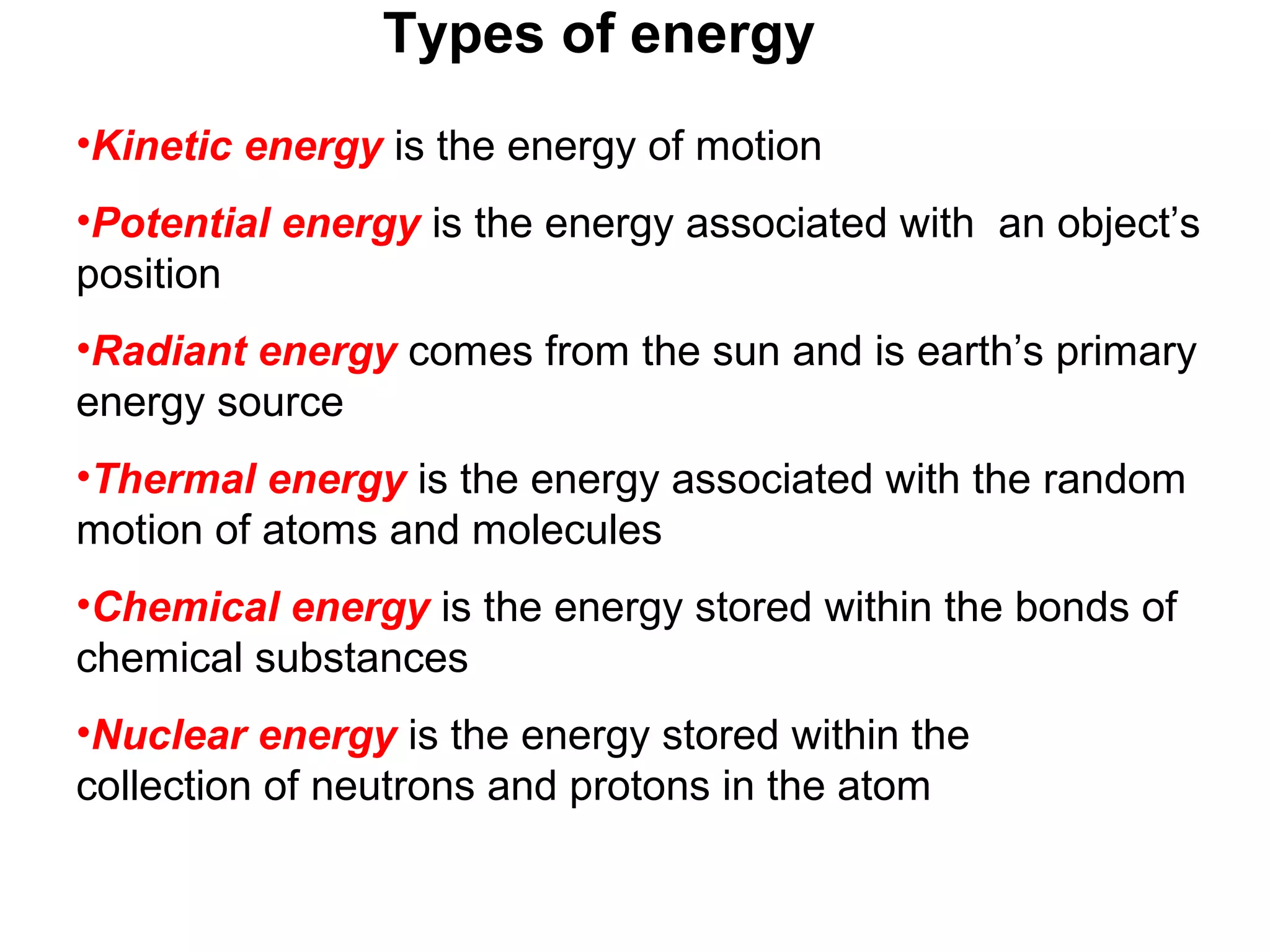
- The six main types of energy - kinetic, potential, radiant, thermal, chemical, and nuclear.
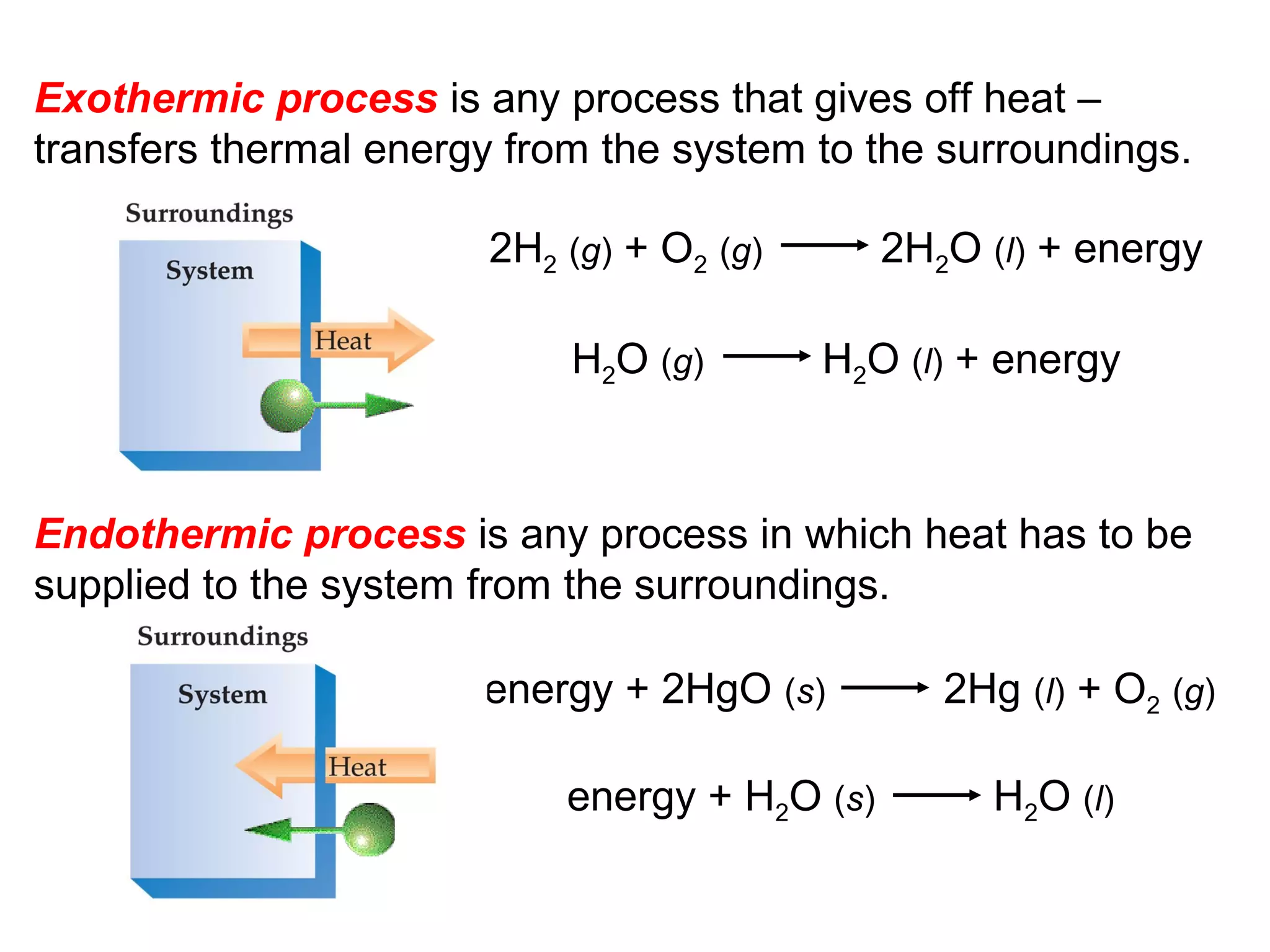
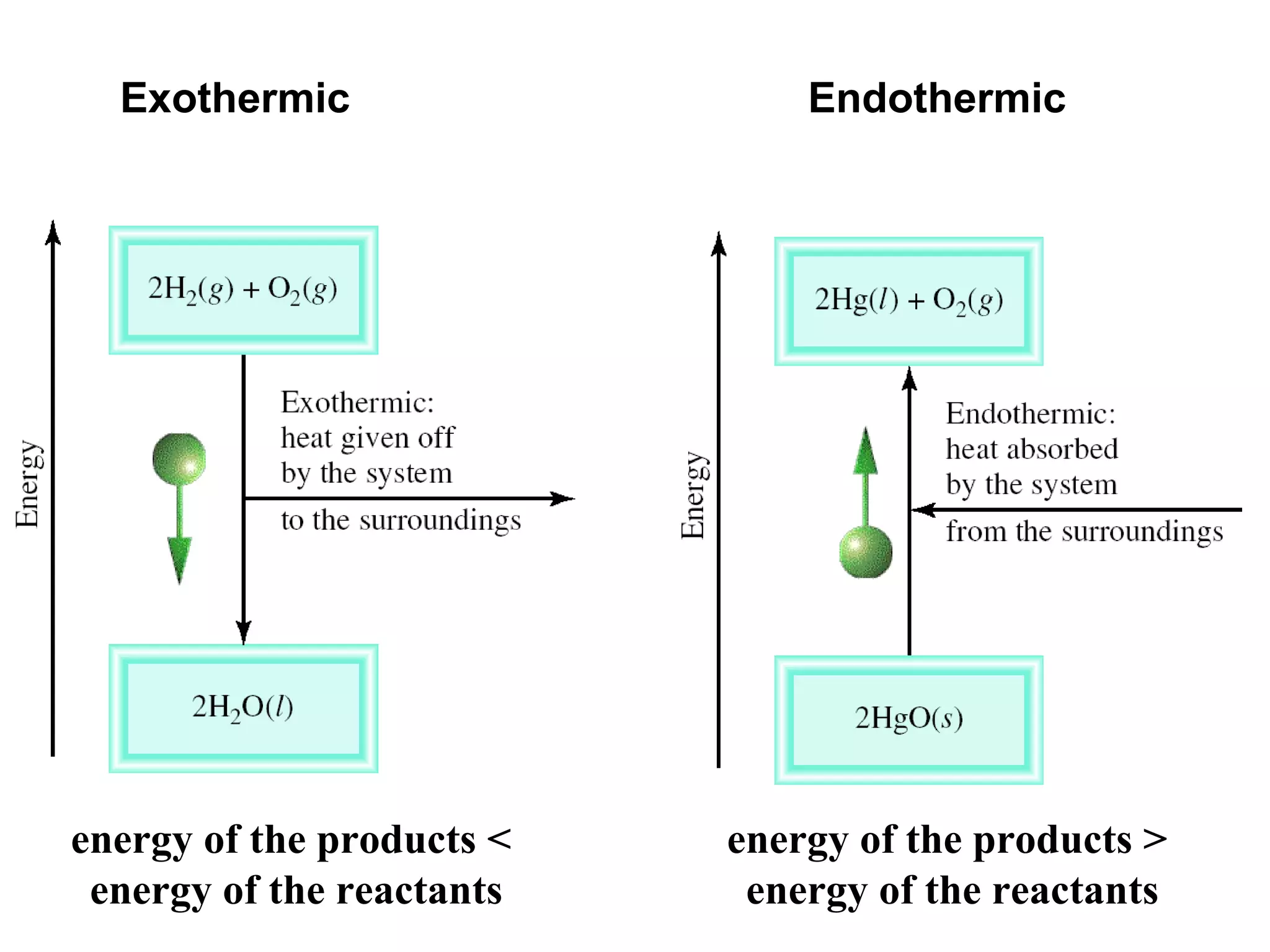
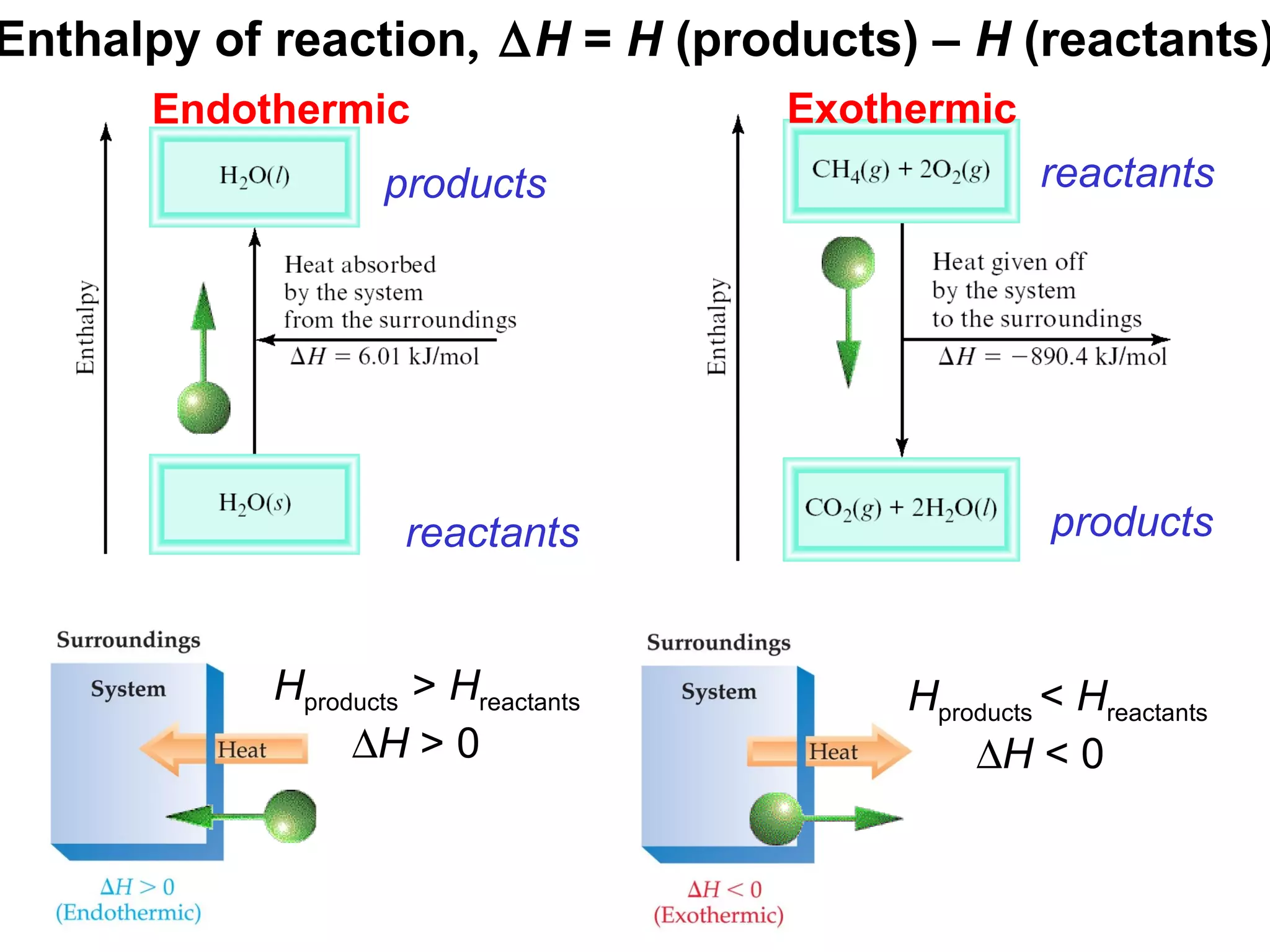
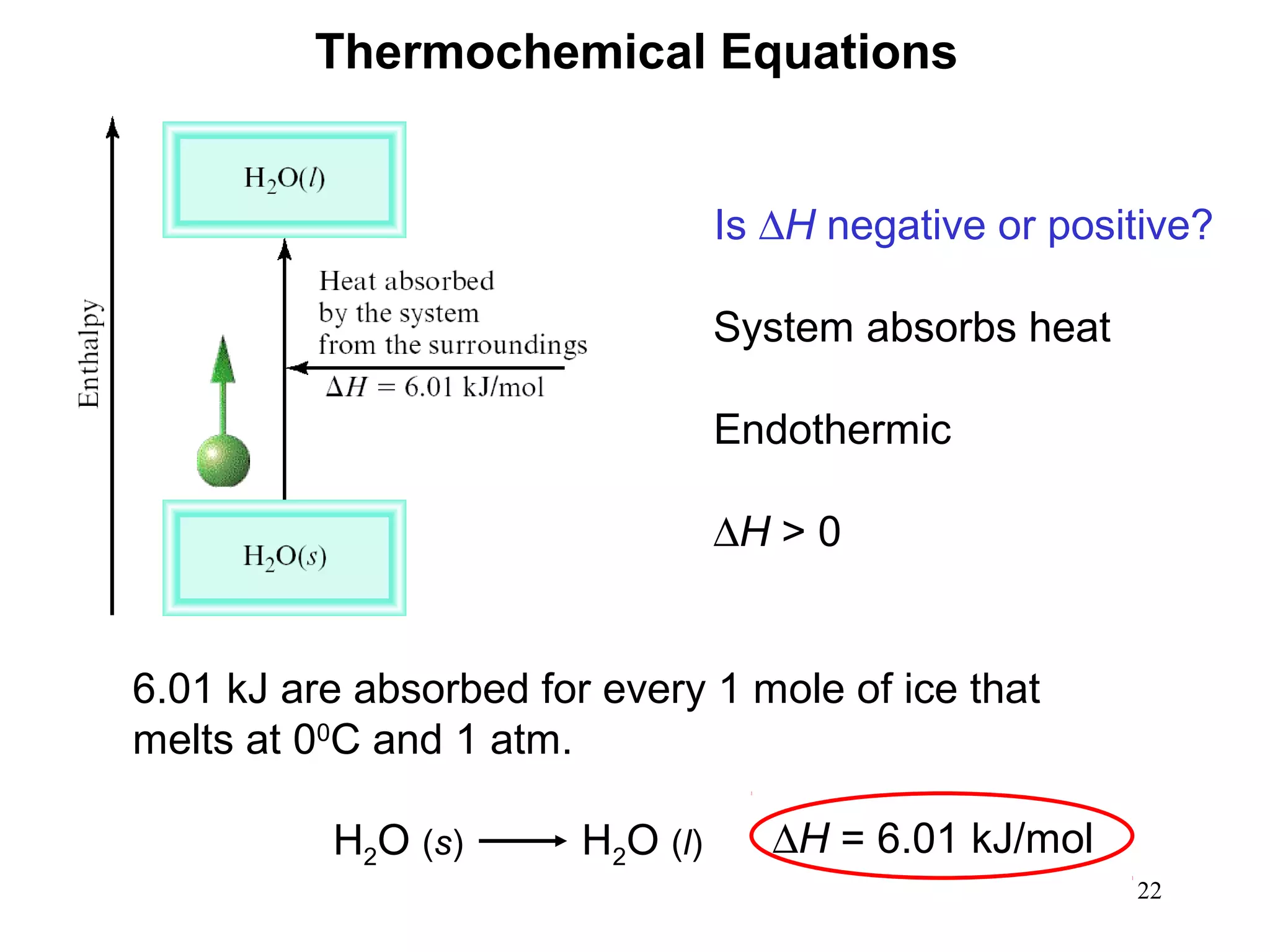
- Exothermic and endothermic reactions, where exothermic reactions release energy as heat and endothermic reactions absorb energy in the form of heat.
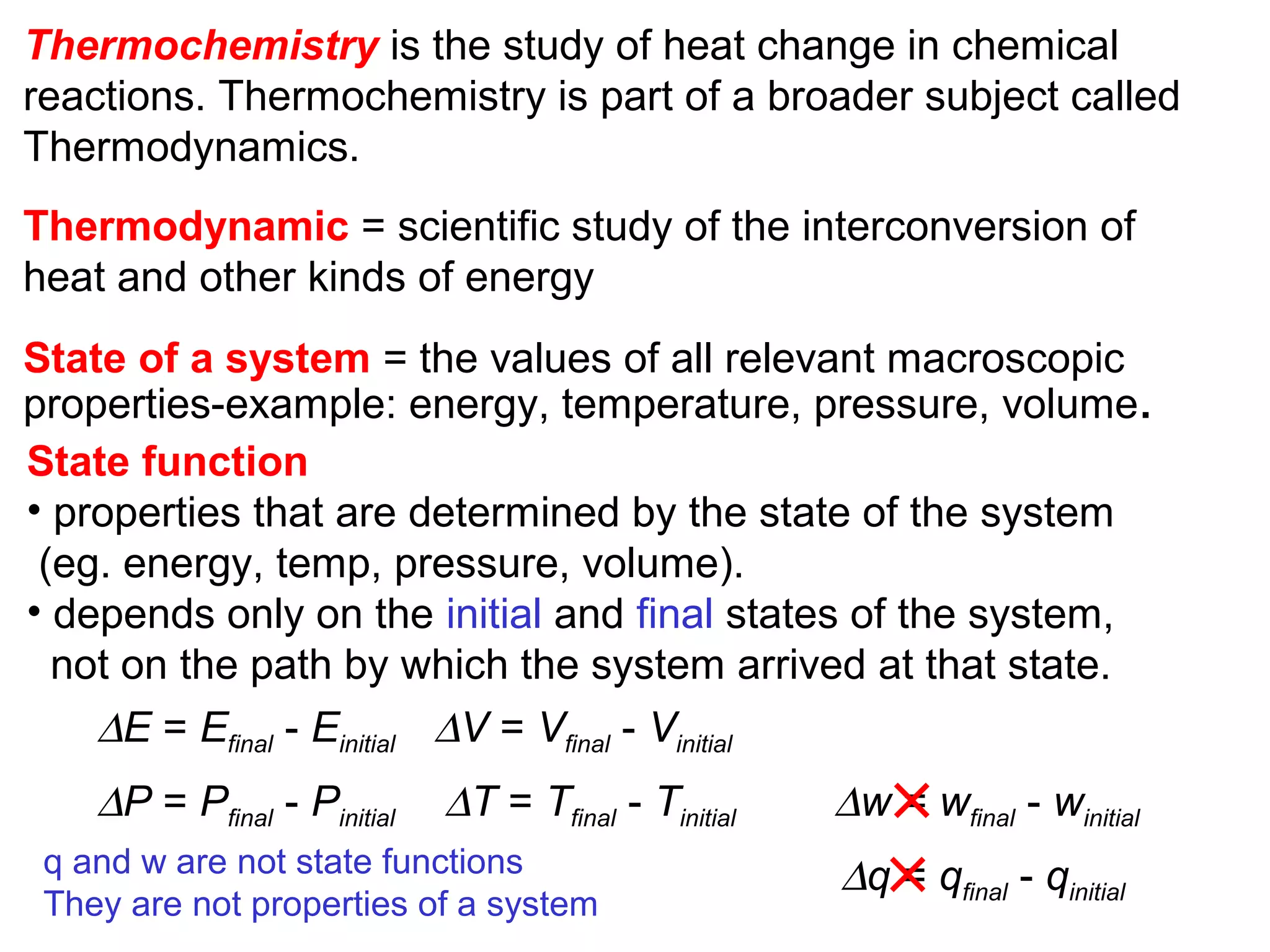
- Thermochemistry and thermodynamics, where thermochemistry is the study of heat changes in chemical reactions and thermodynamics is the broader study of energy interconversions.
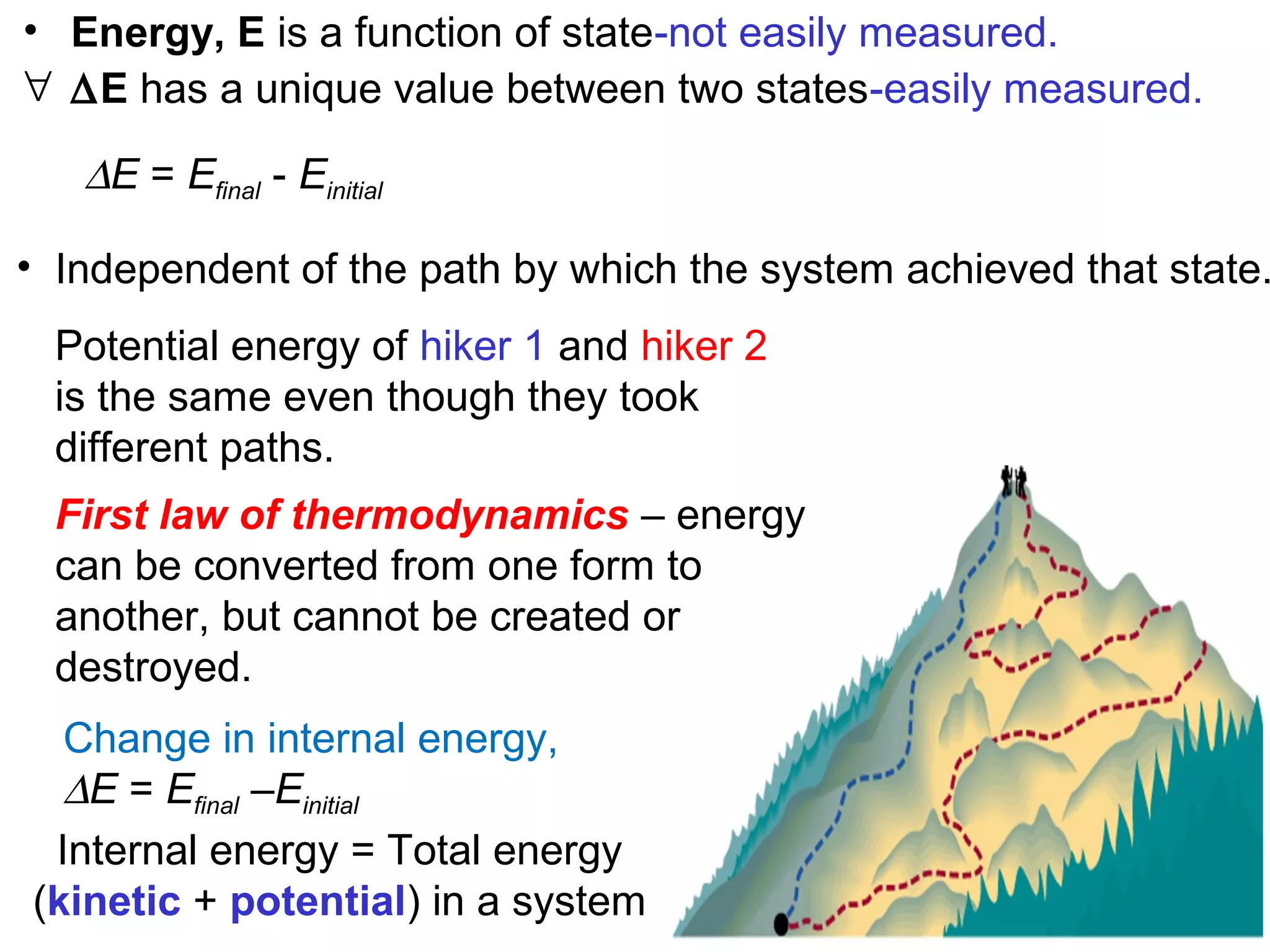
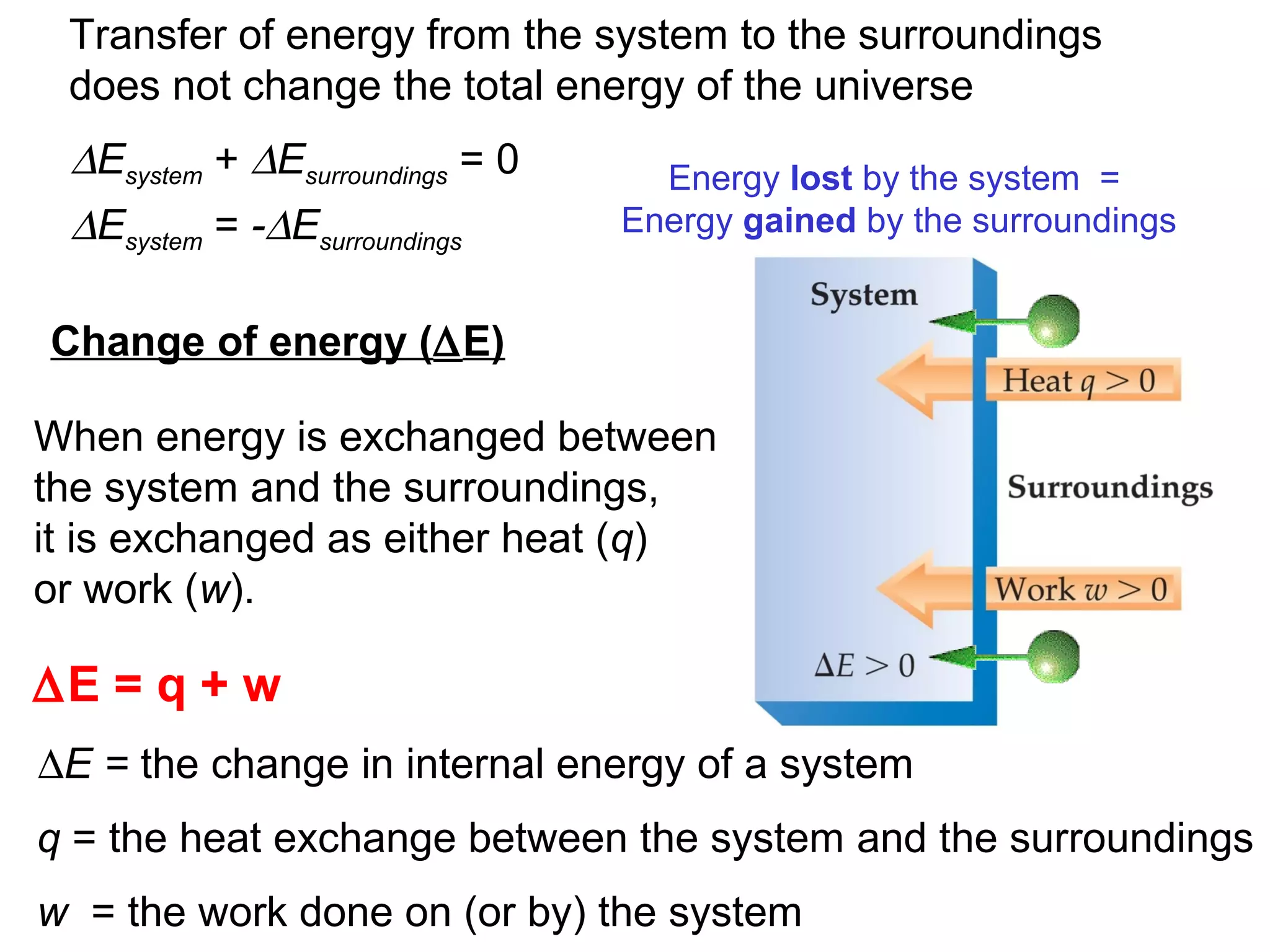
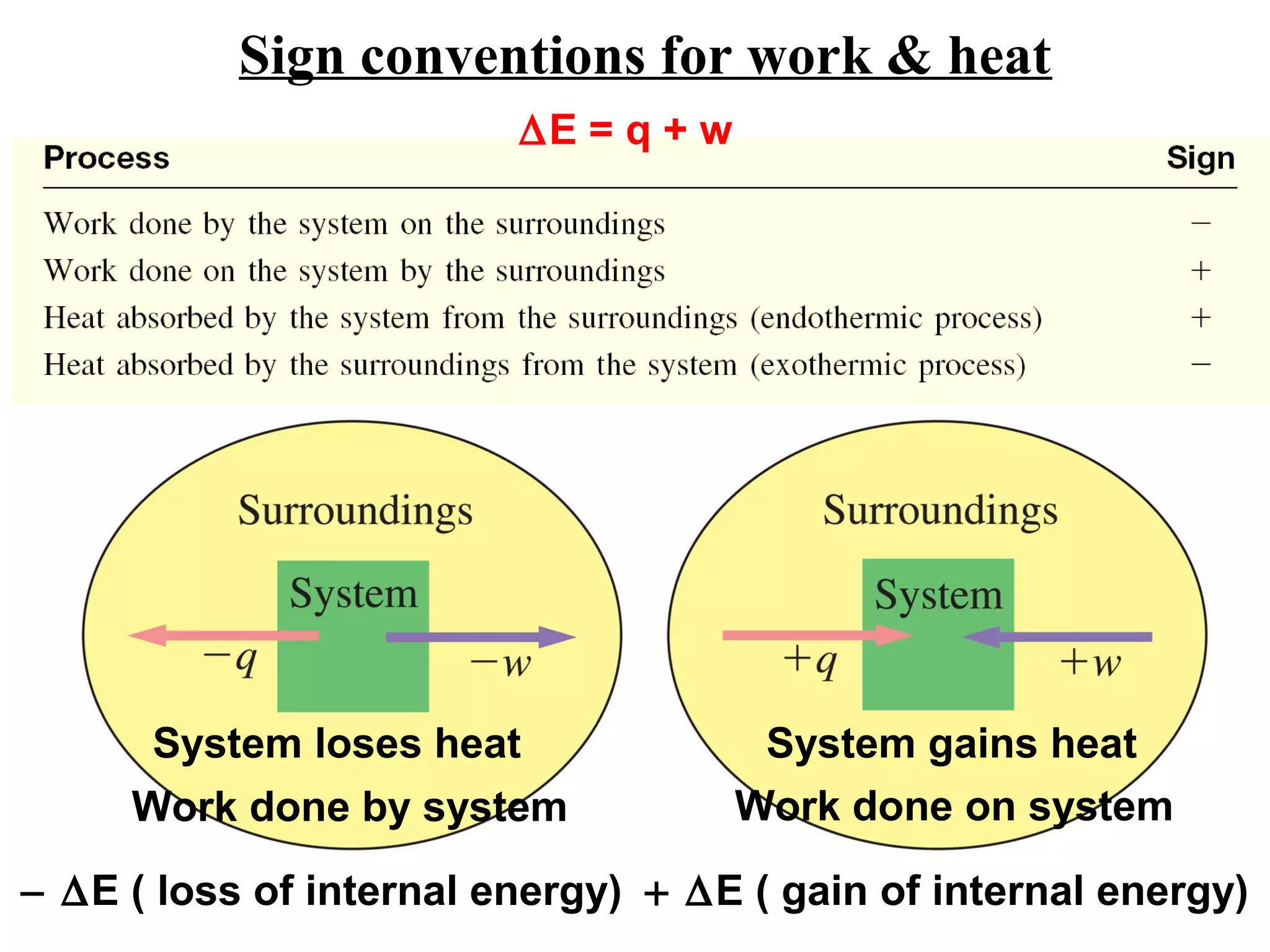
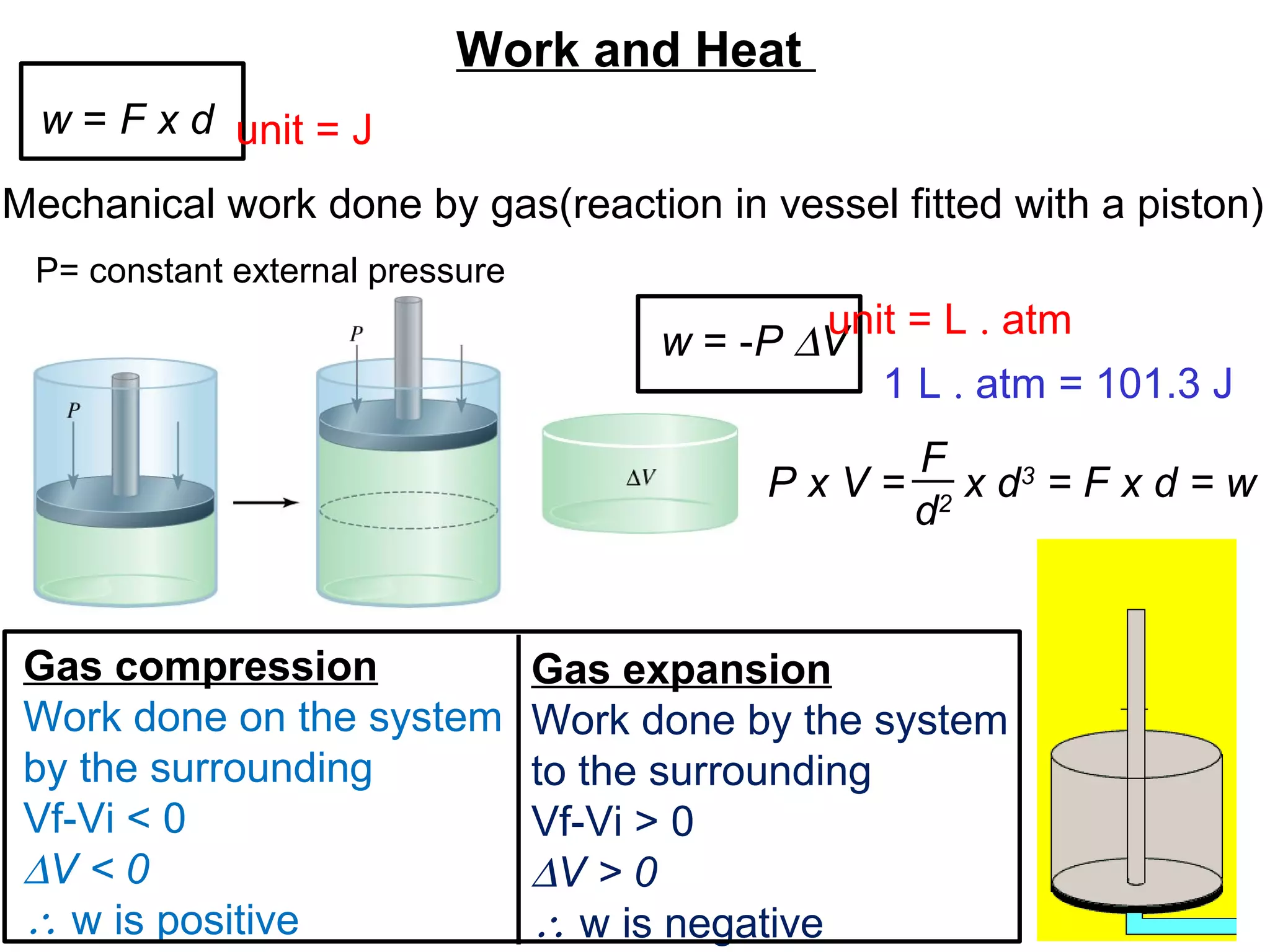
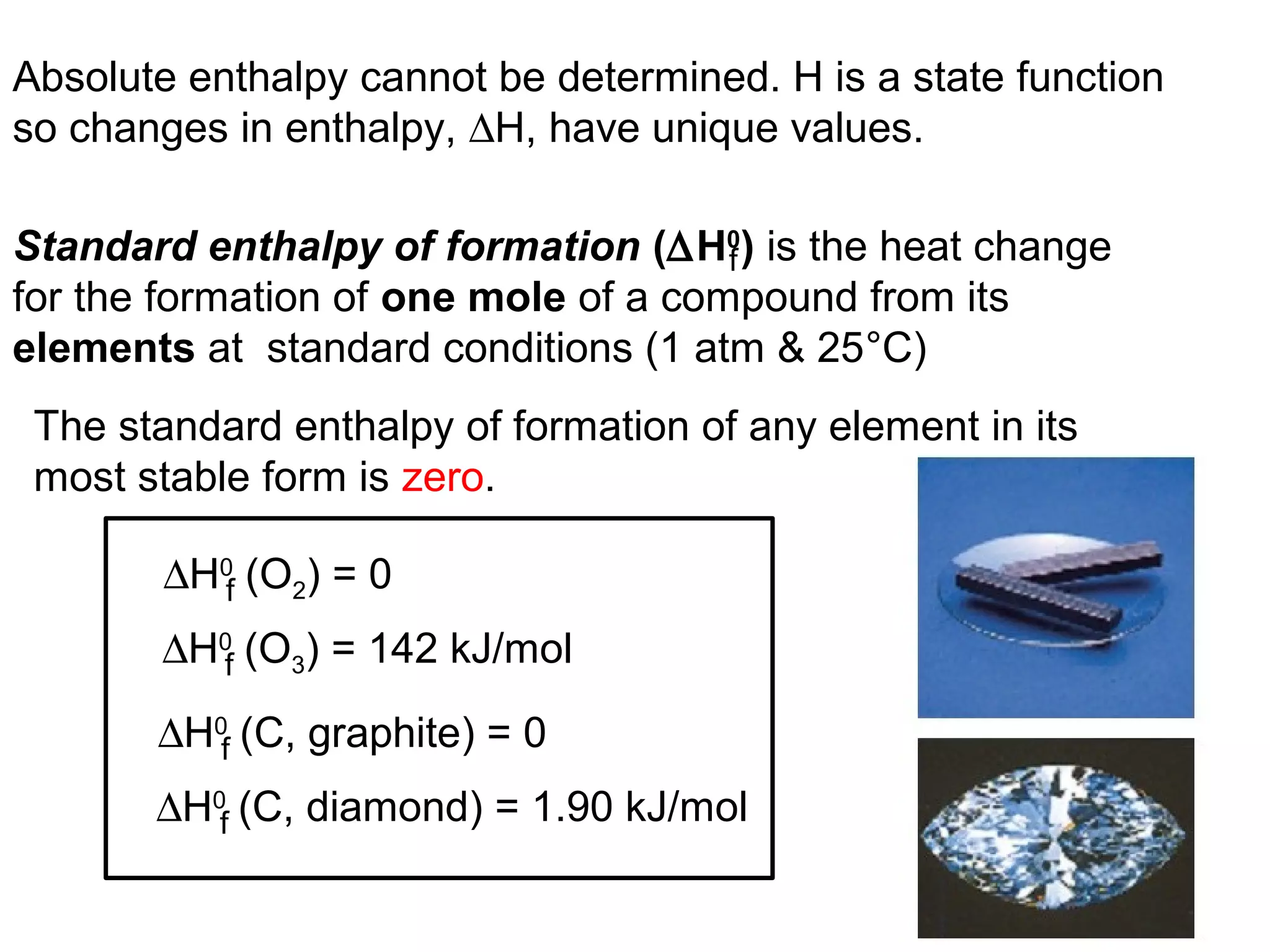
- Key thermodynamic concepts like state functions, the first law of thermodynamics, enthalpy, and standard enthalpy





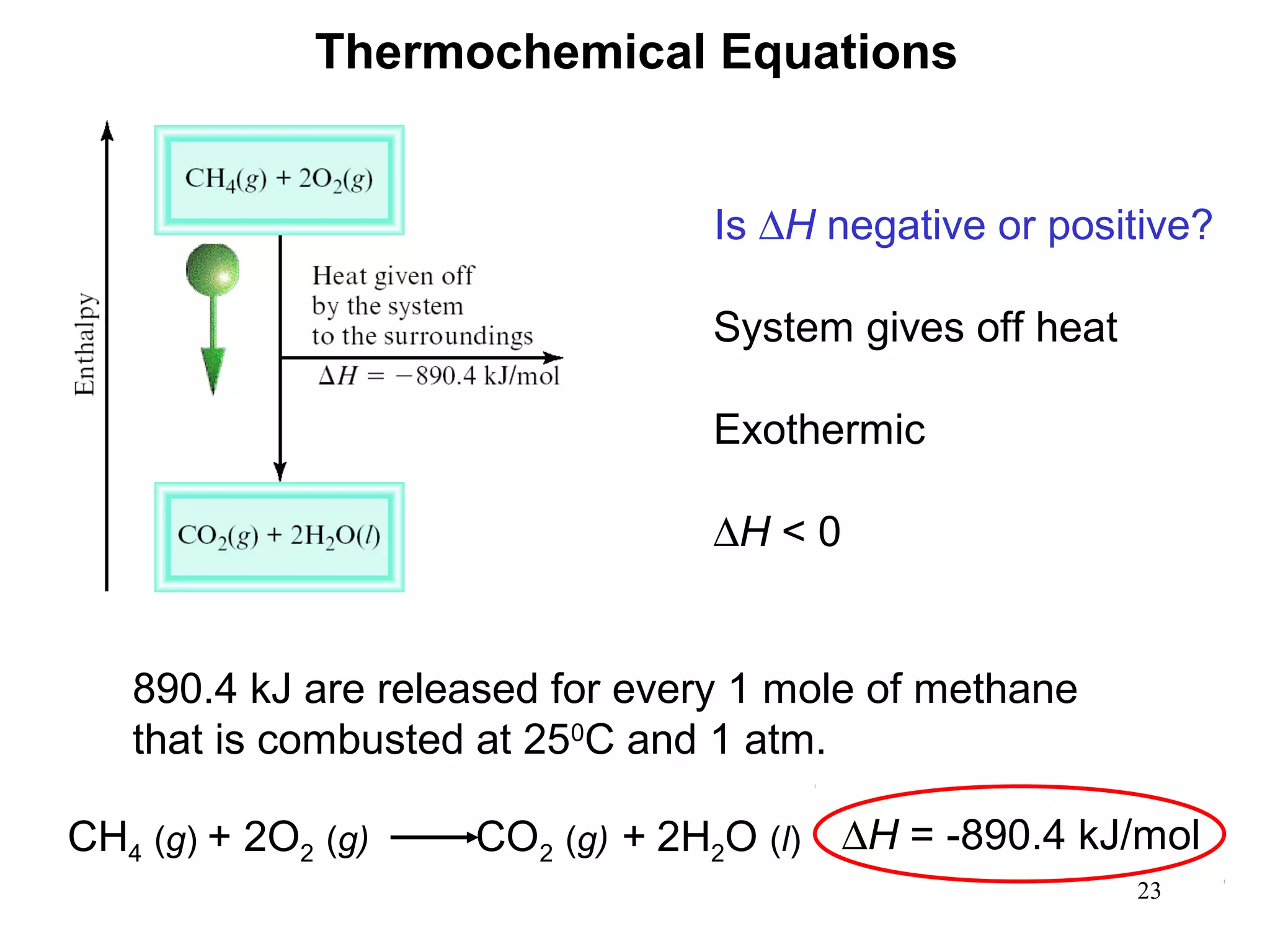
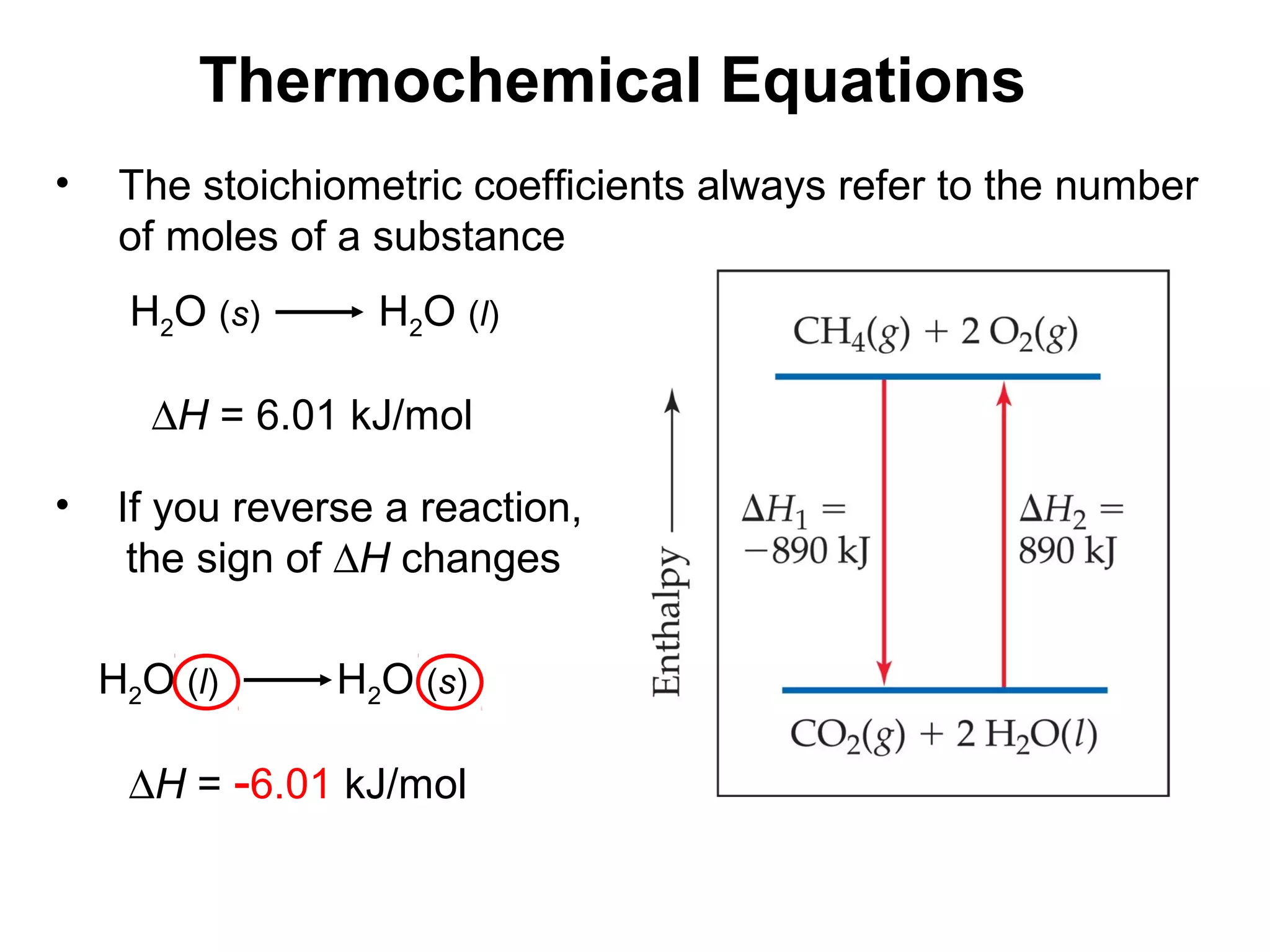
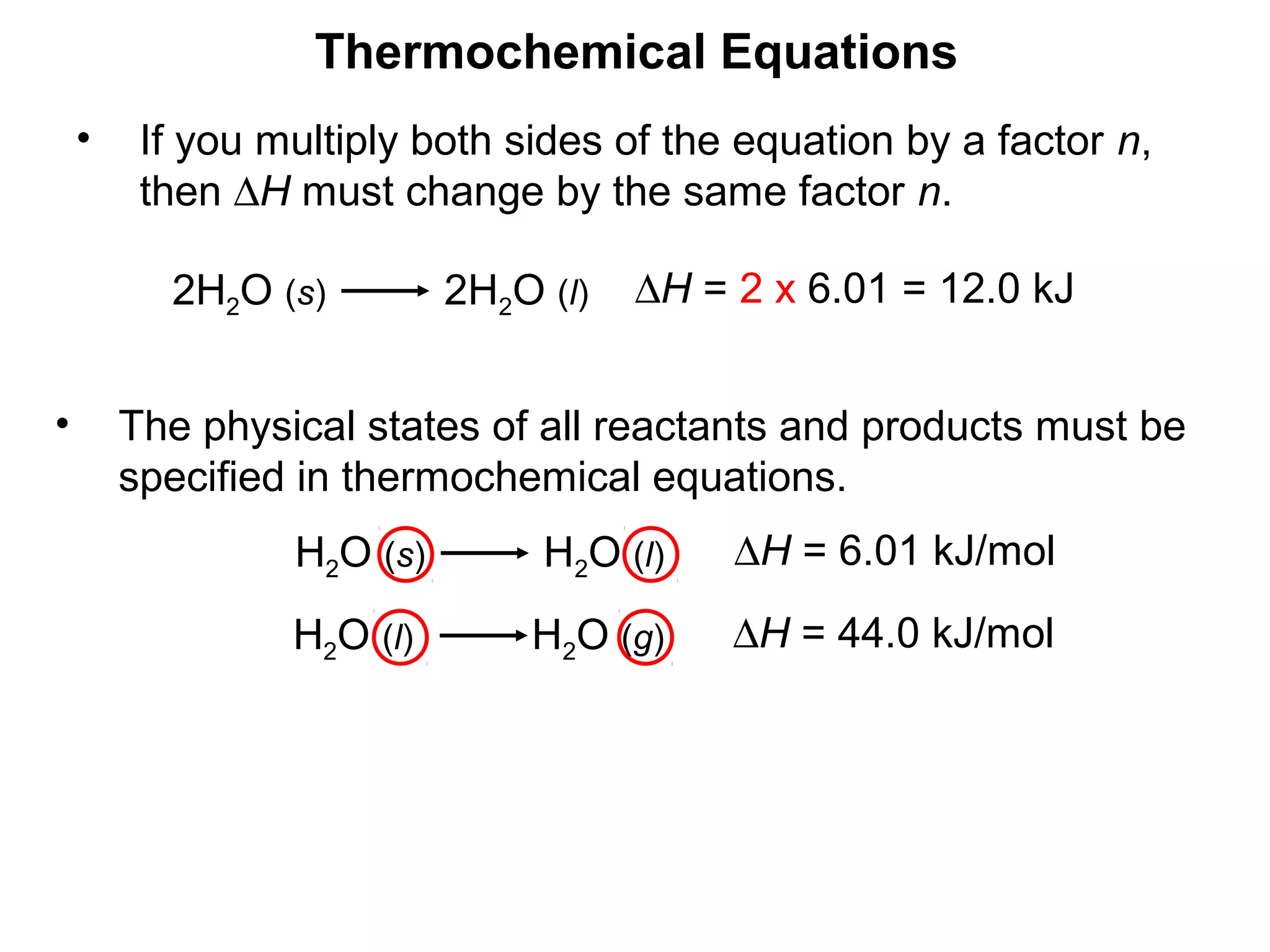
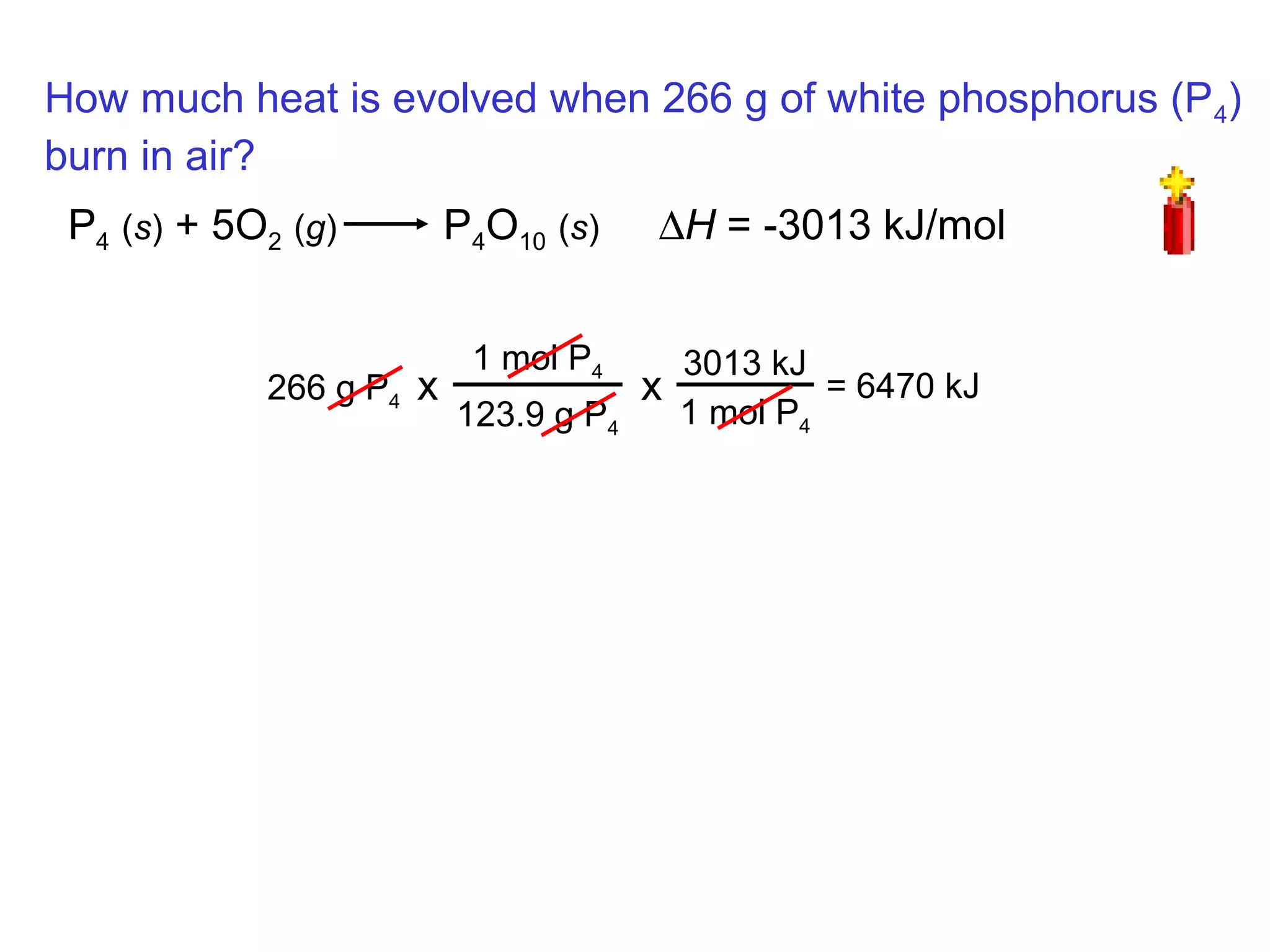
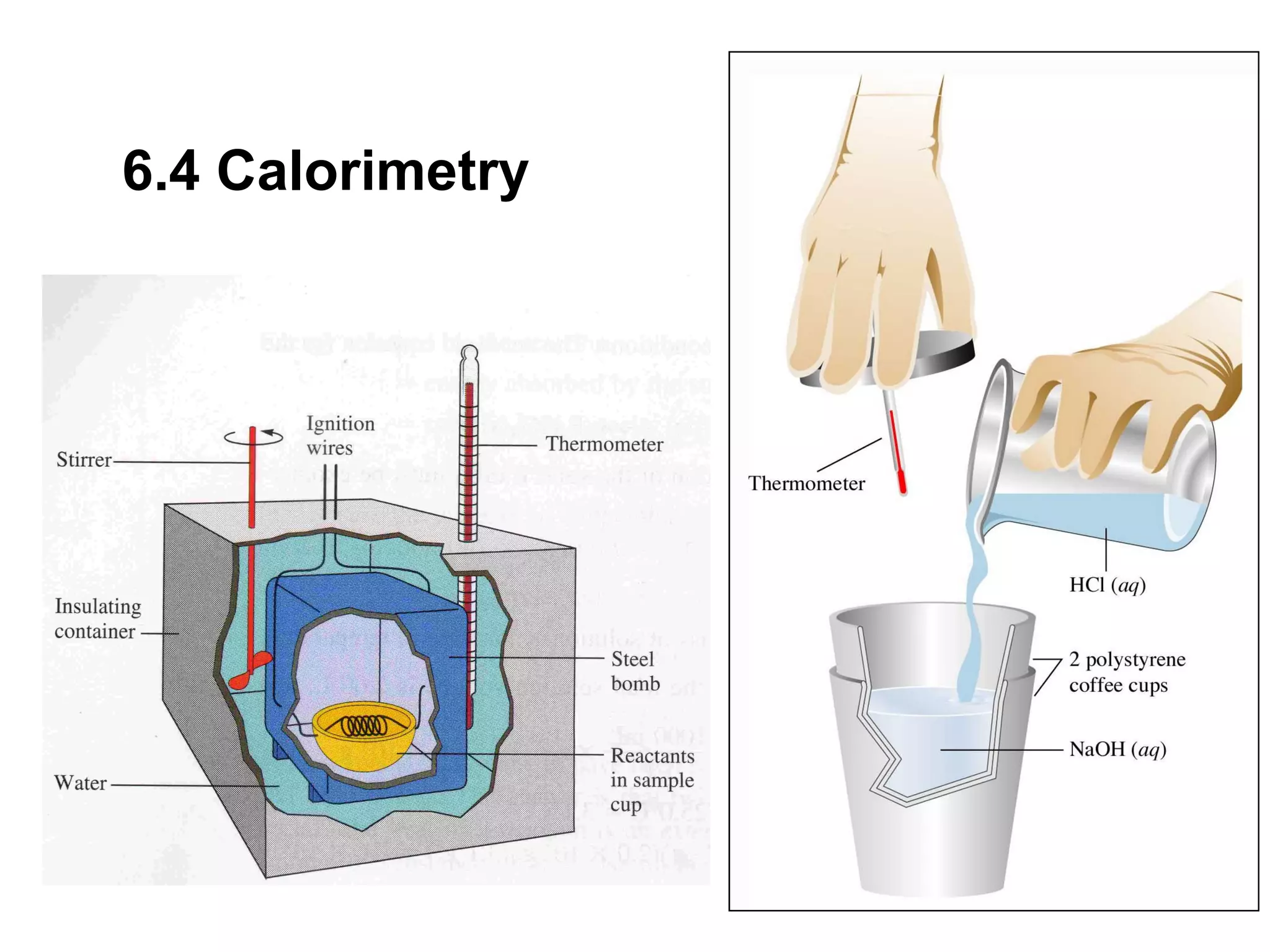
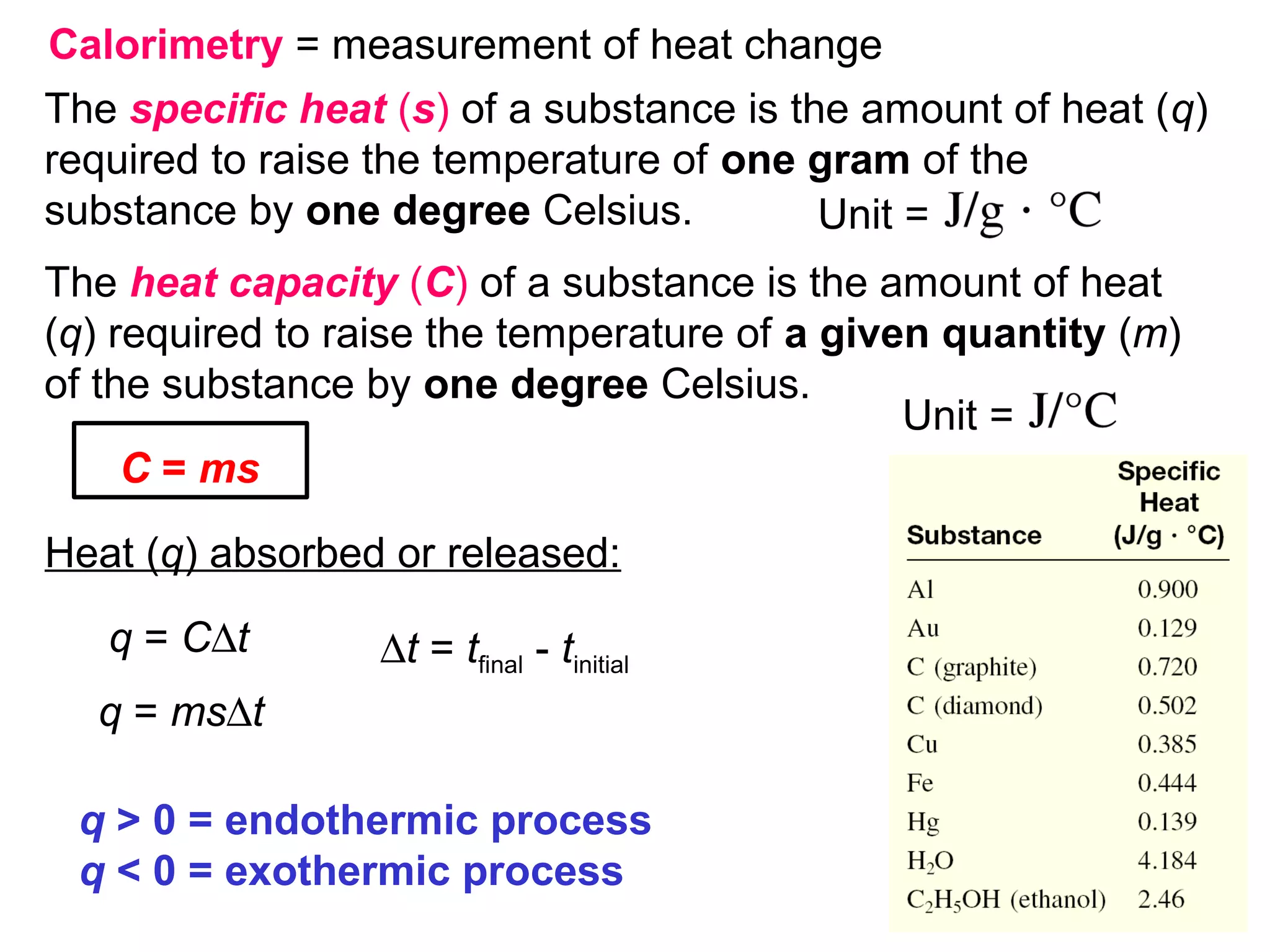
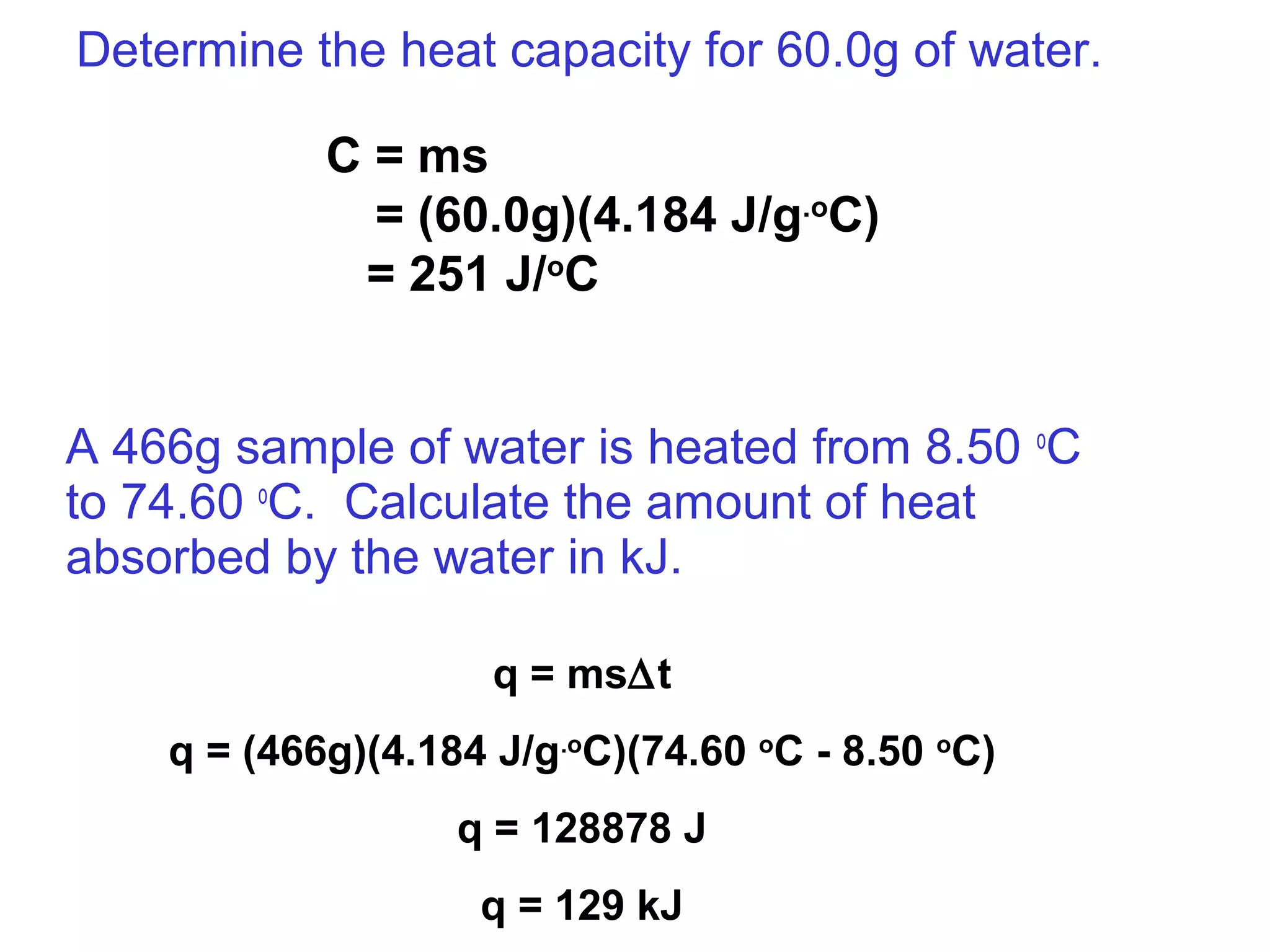
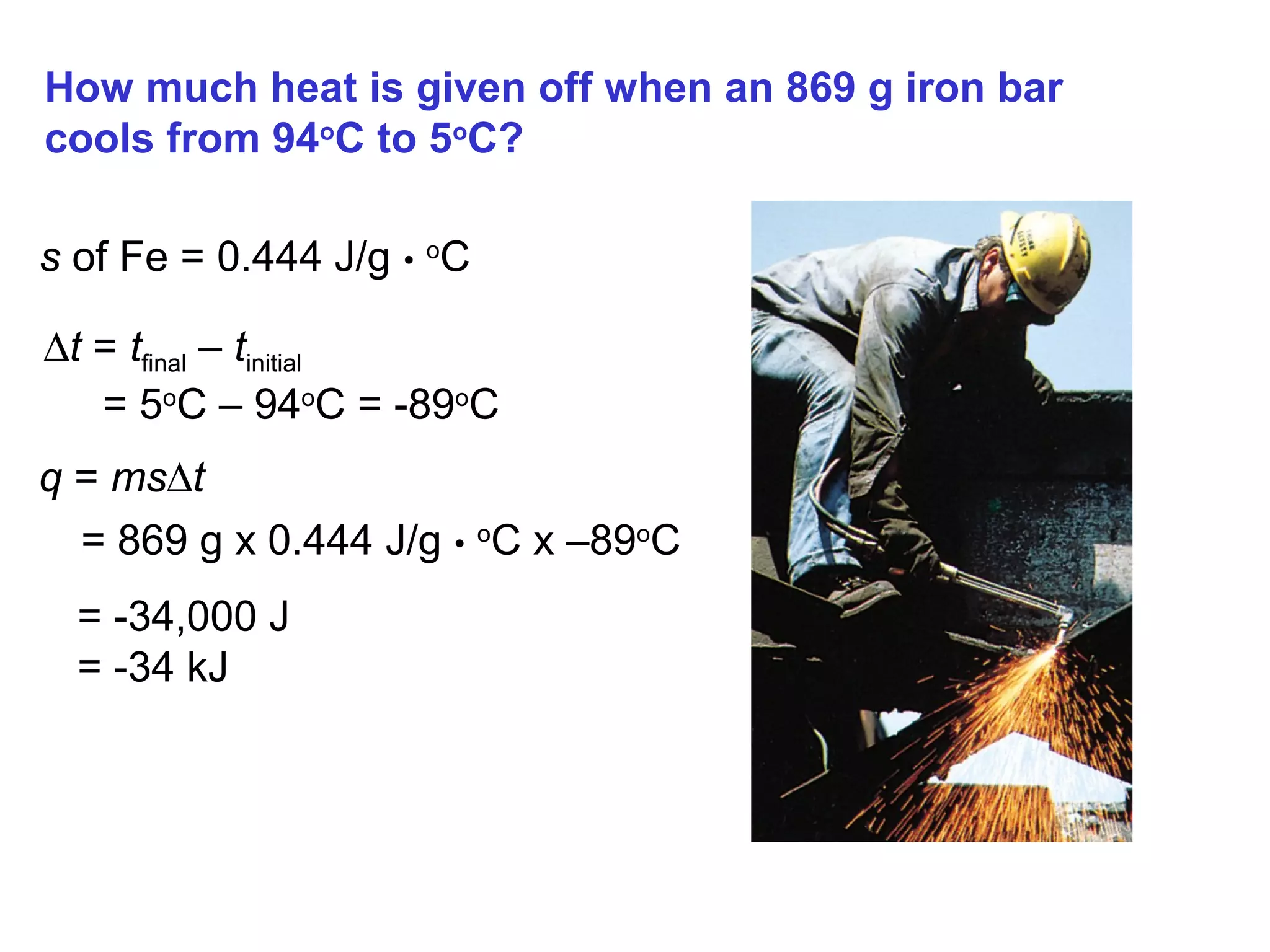
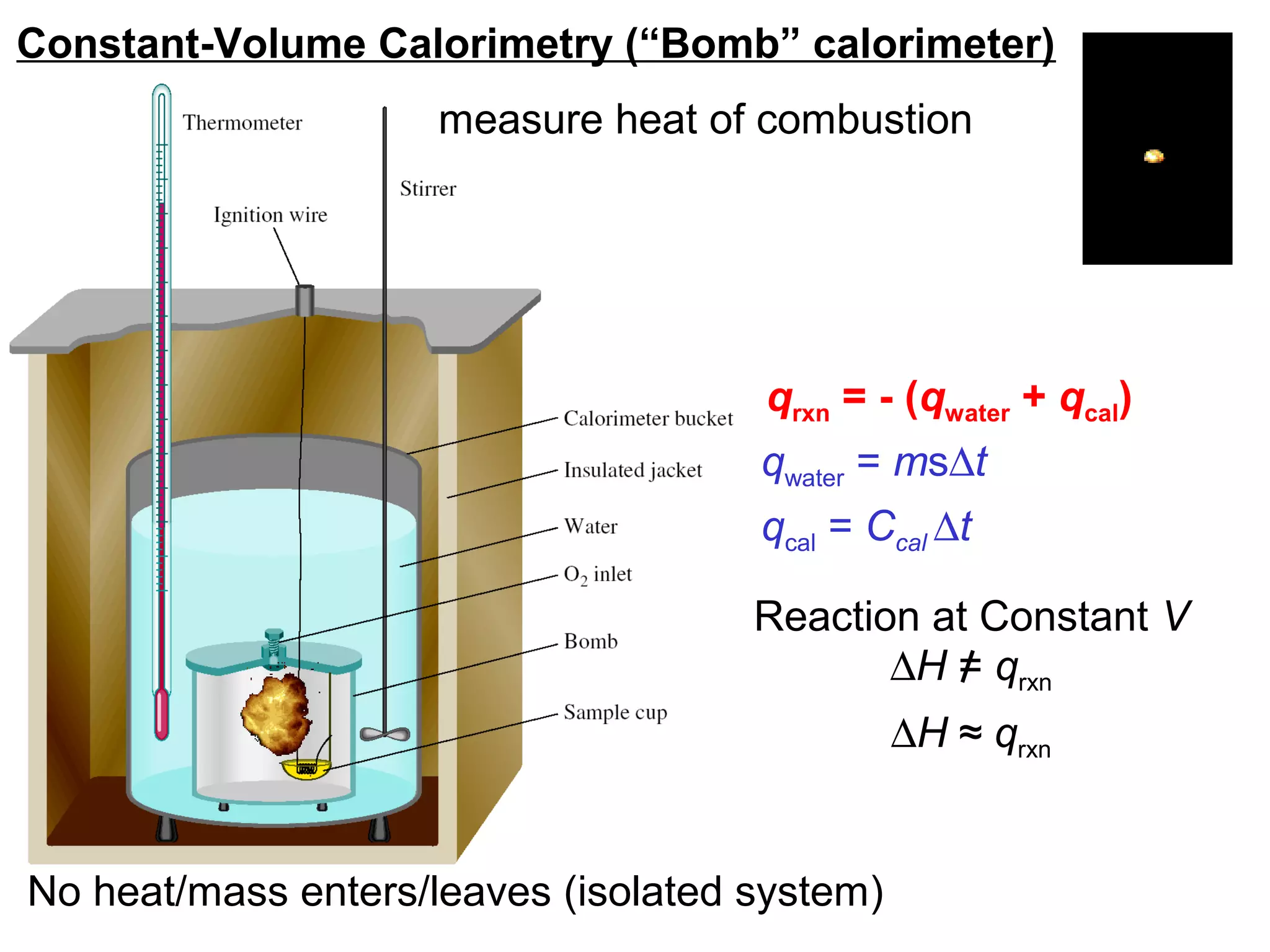
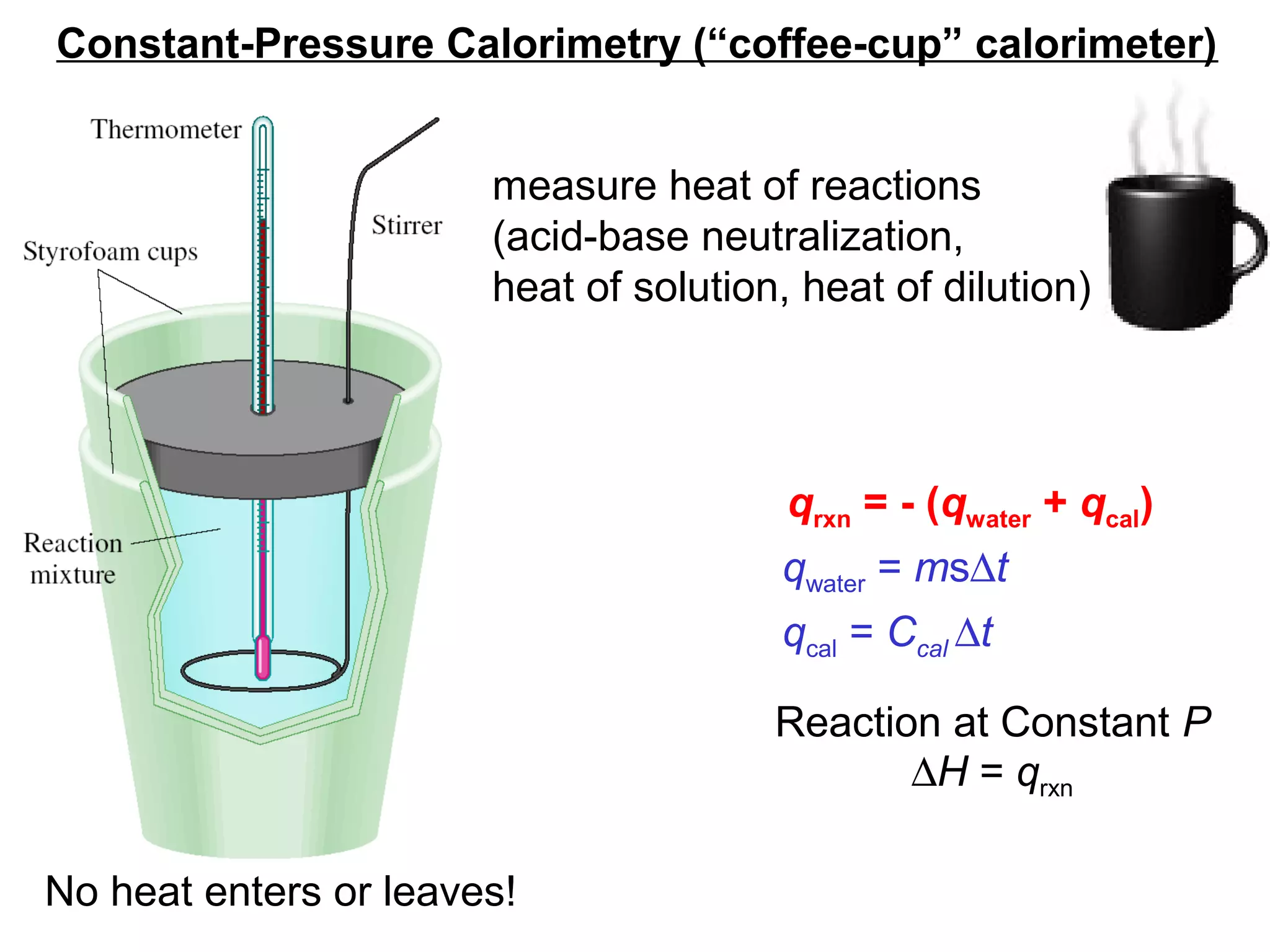
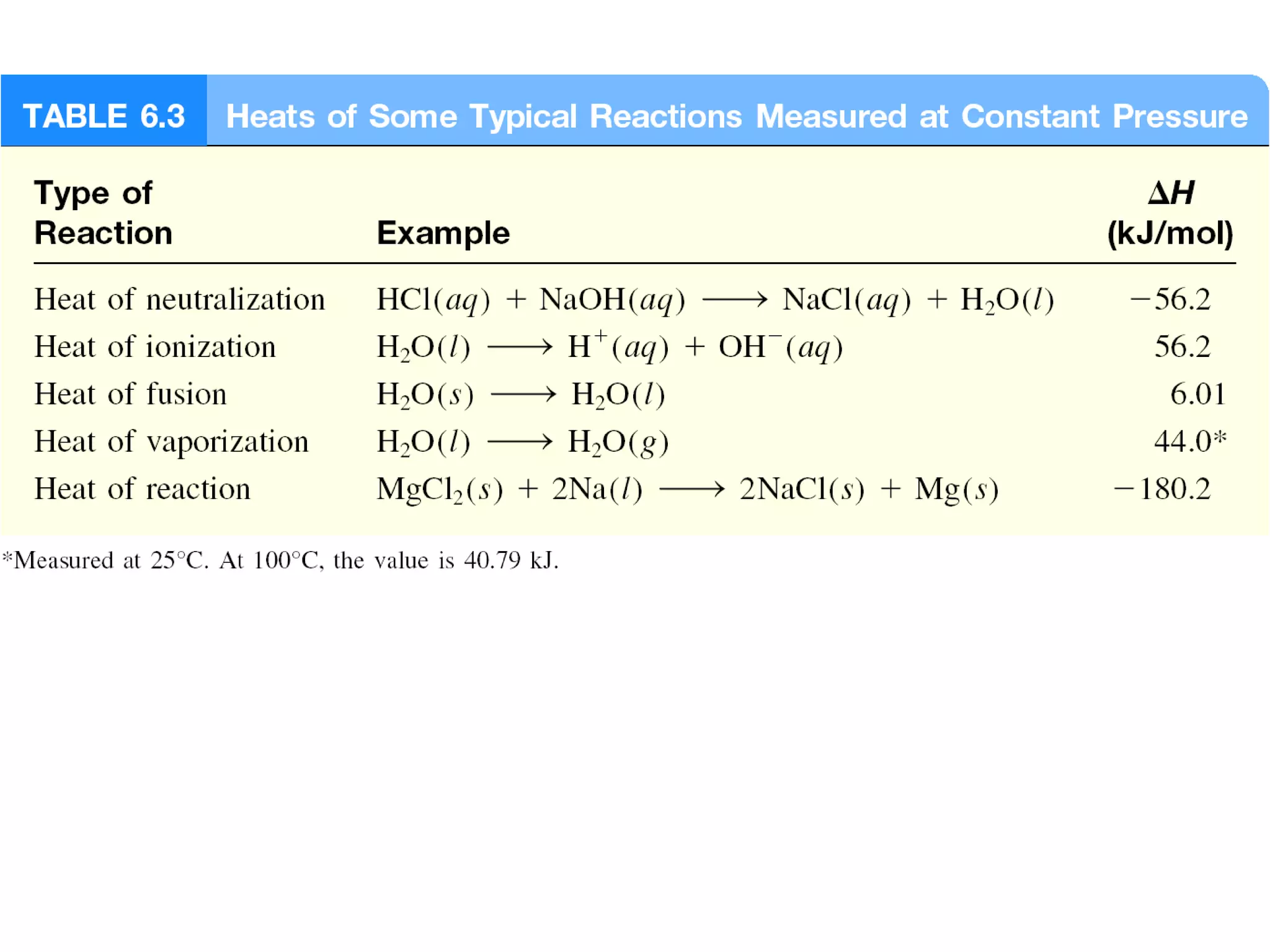
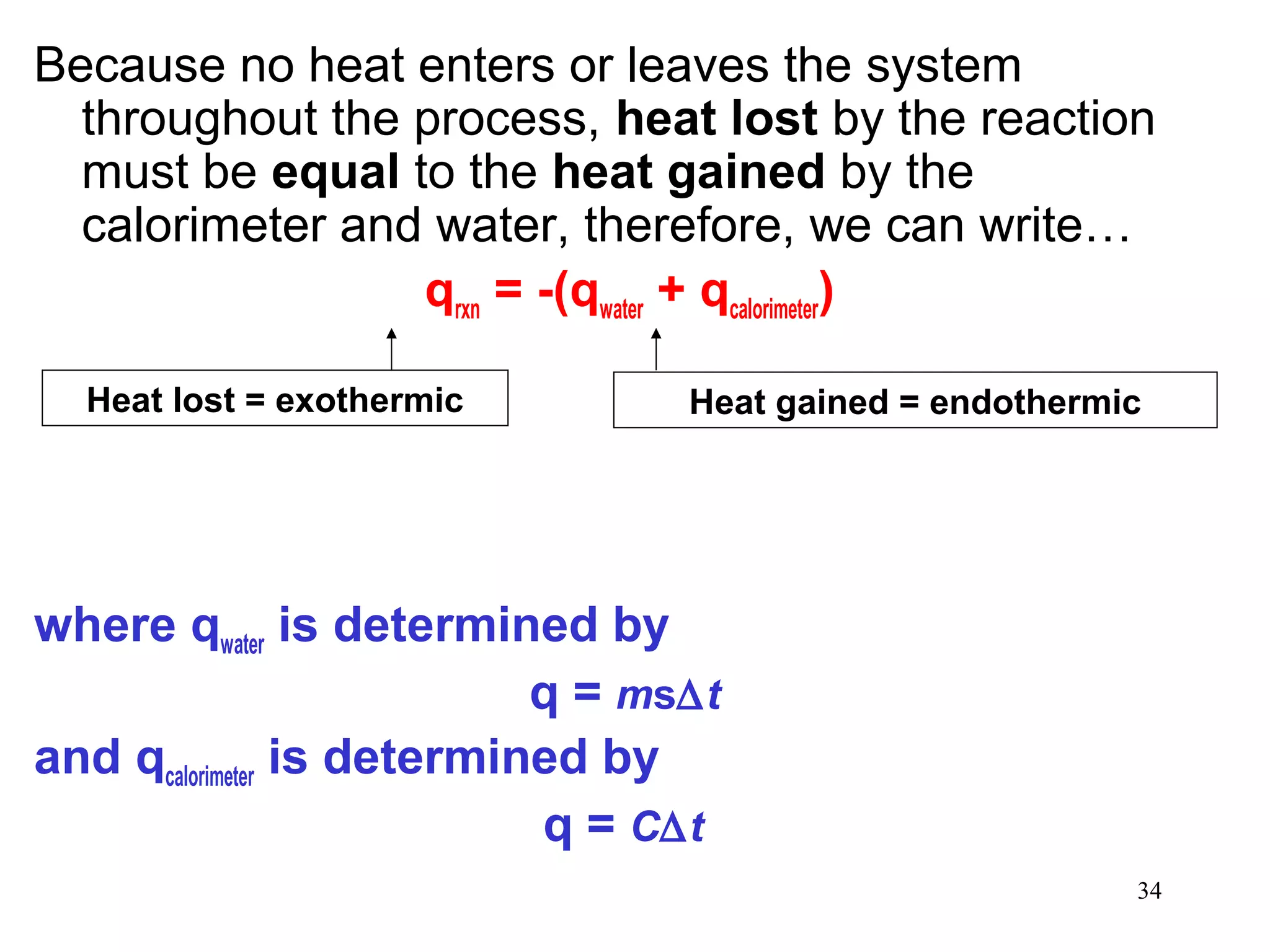

![The standard enthalpy of reaction (∆H0
) is the enthalpy of
a reaction carried out at 1 atm.
rxn
aA + bB cC + dD
∆H0
rxn d∆H0
(D)fc∆H0
(C)f= [ + ] - b∆H0
(B)fa∆H0
(A)f[ + ]
∆H0
rxn n∆H0
(products)f= Σ m∆H0
(reactants)fΣ-
∆H0
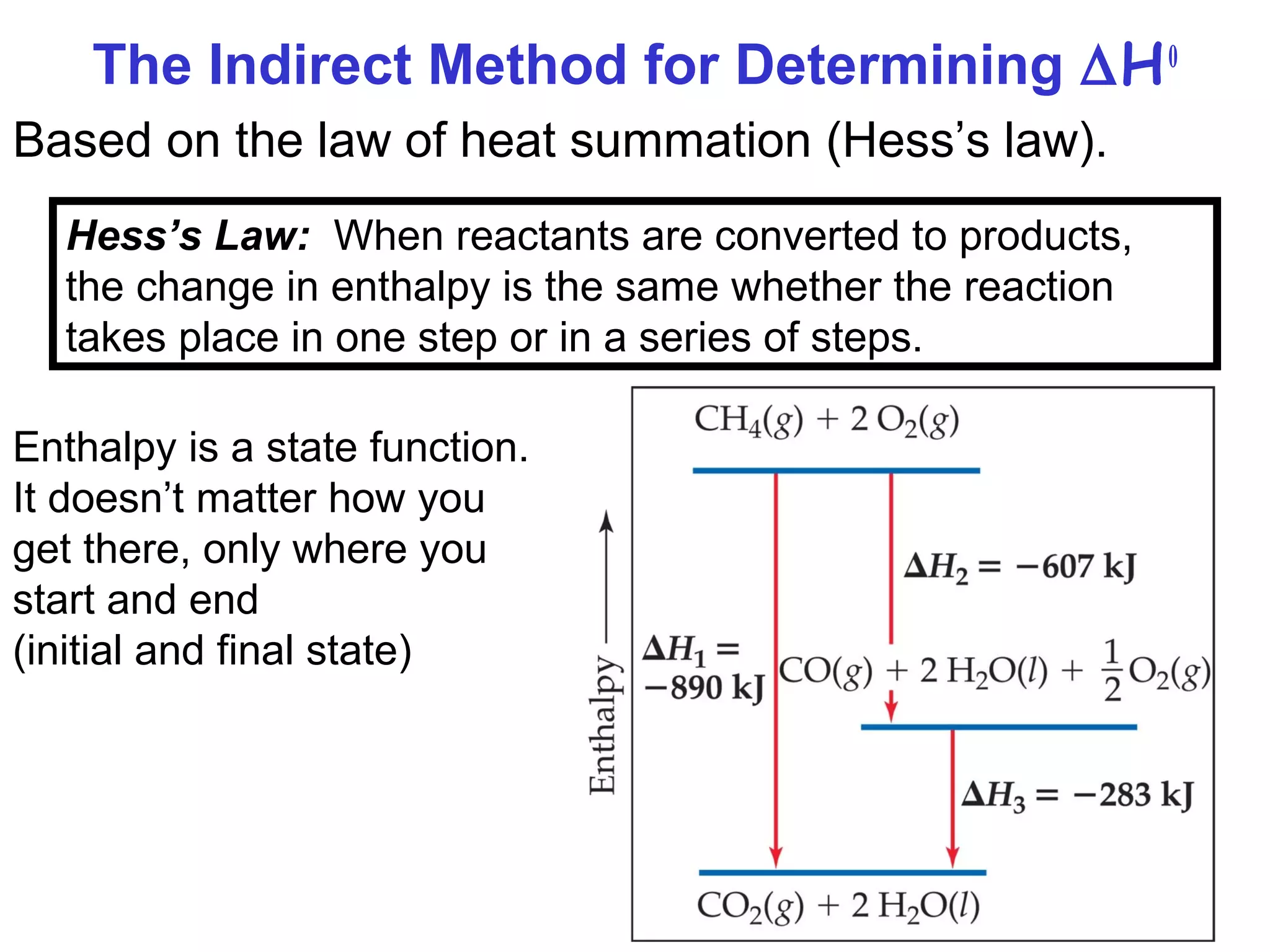
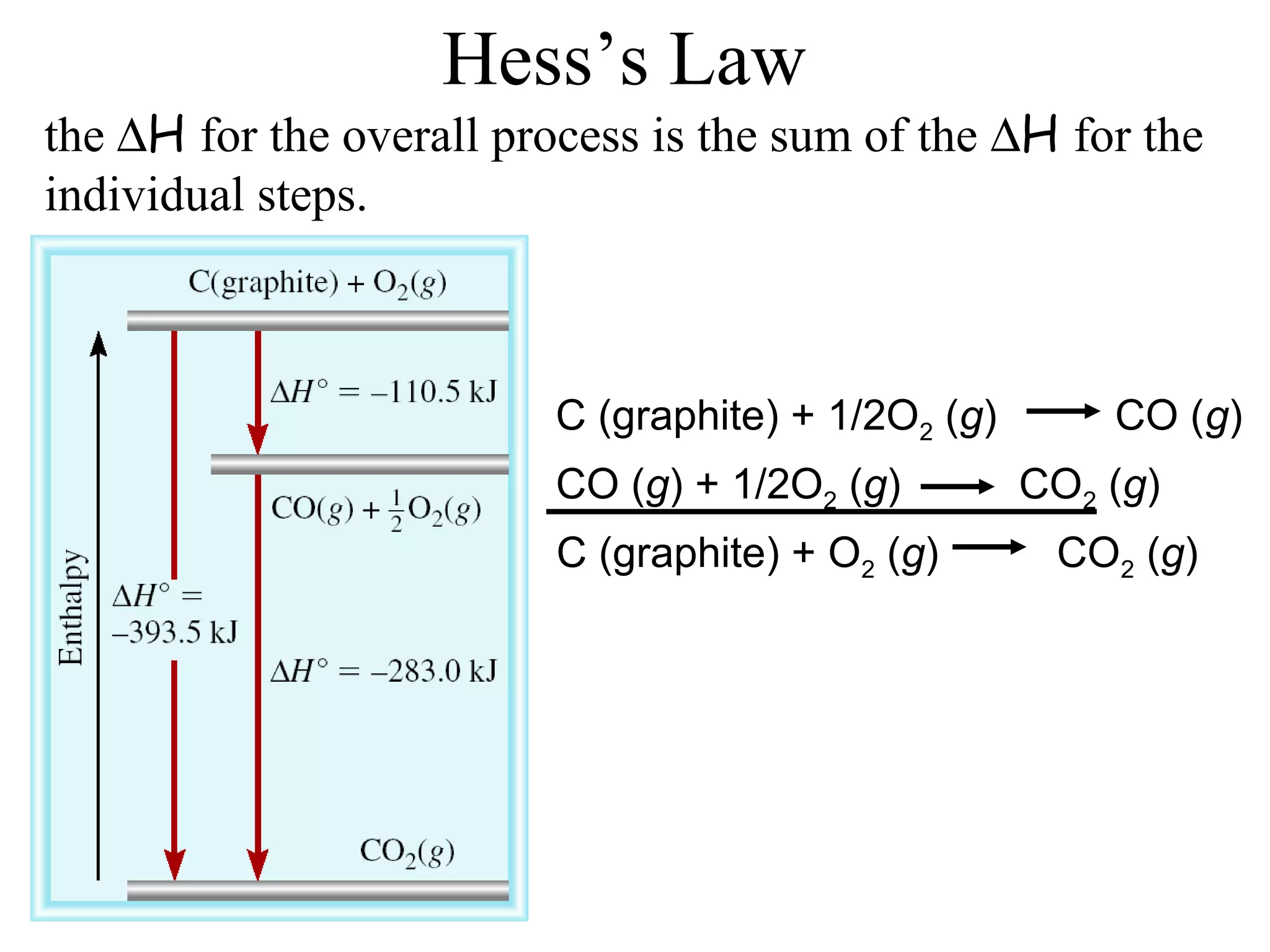
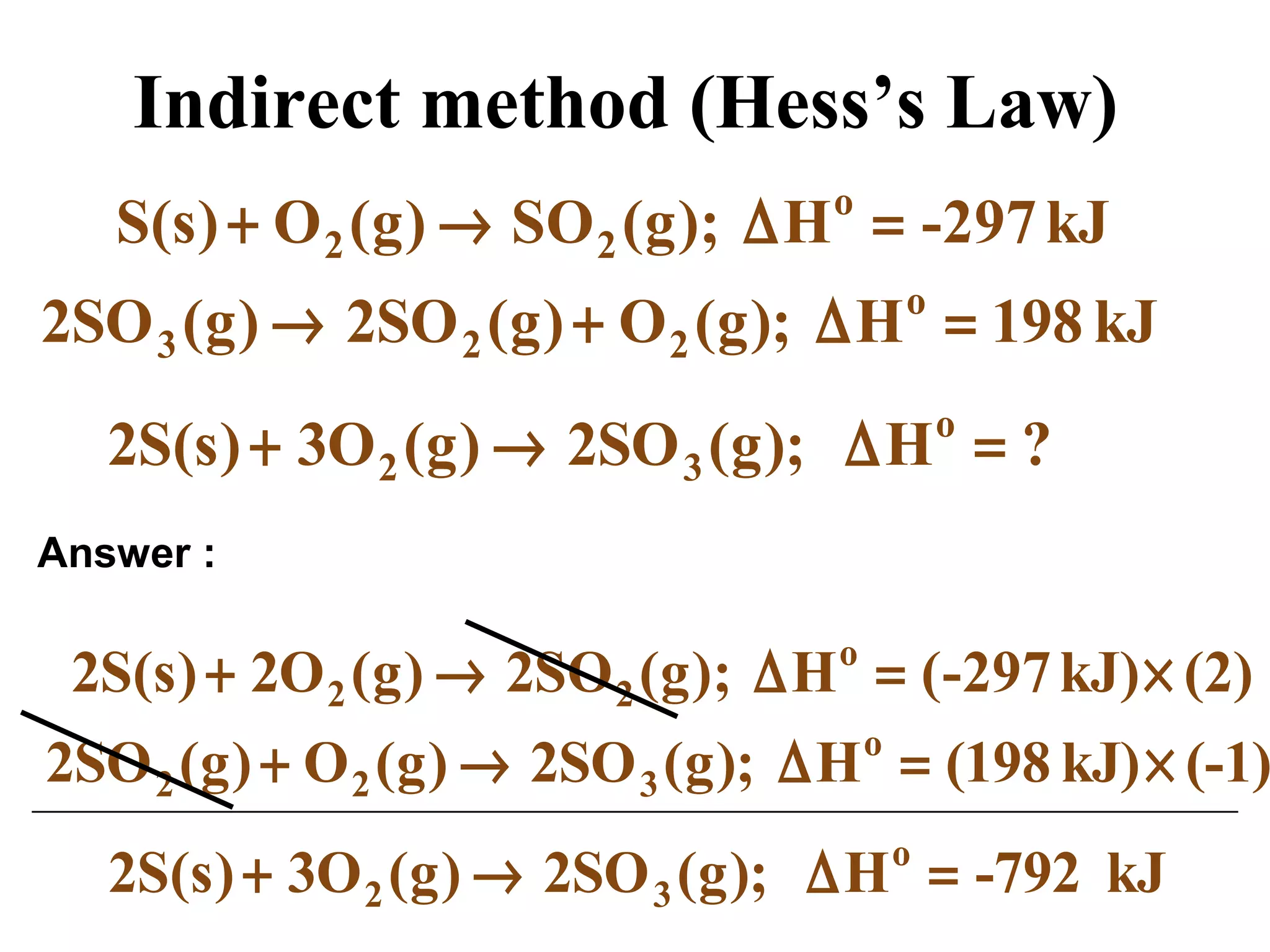
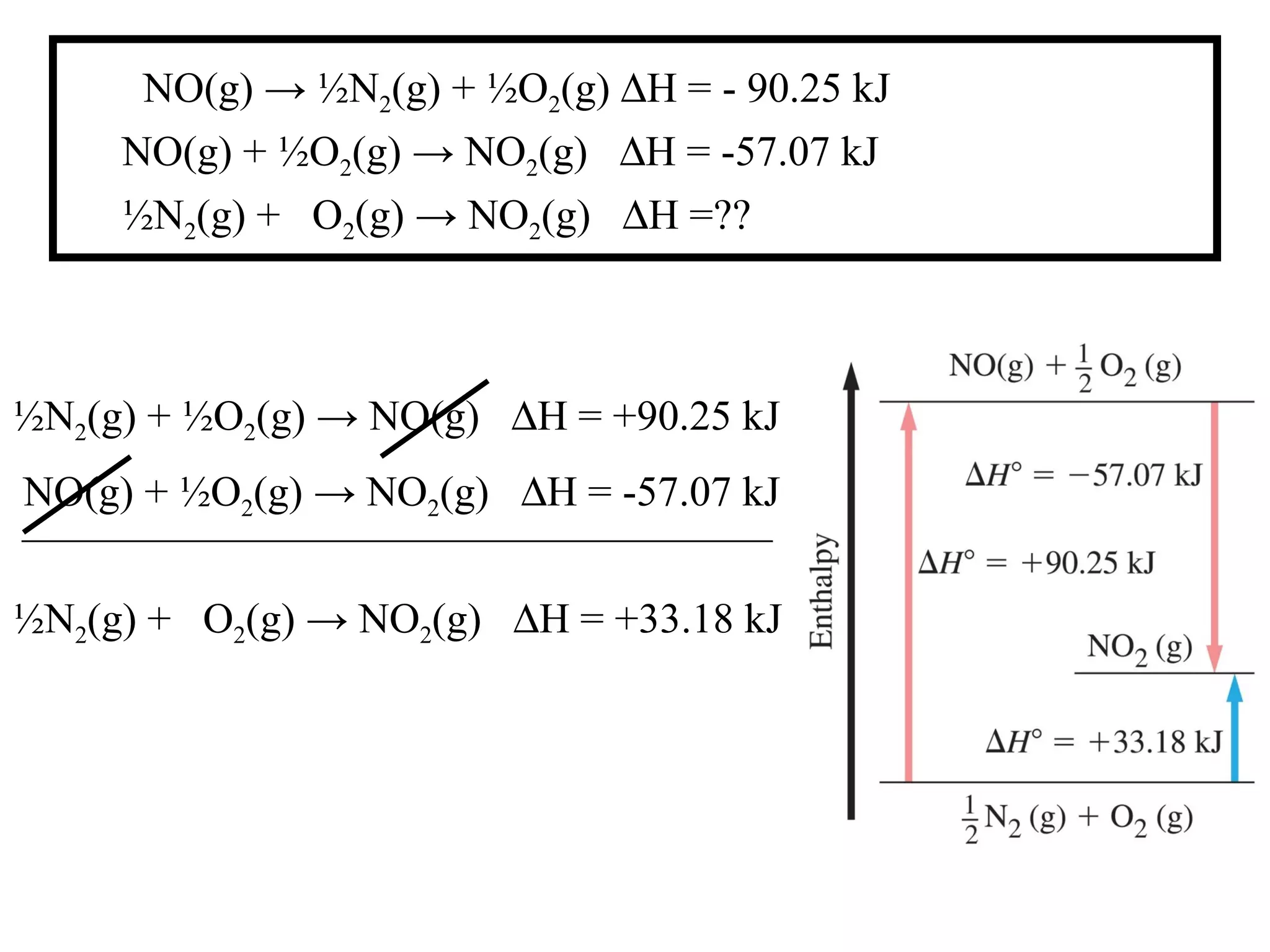
can be determined using the direct method or
the indirect method.](https://image.slidesharecdn.com/chapter6notes-150901140908-lva1-app6892/75/Chapter-6-notes-39-2048.jpg)
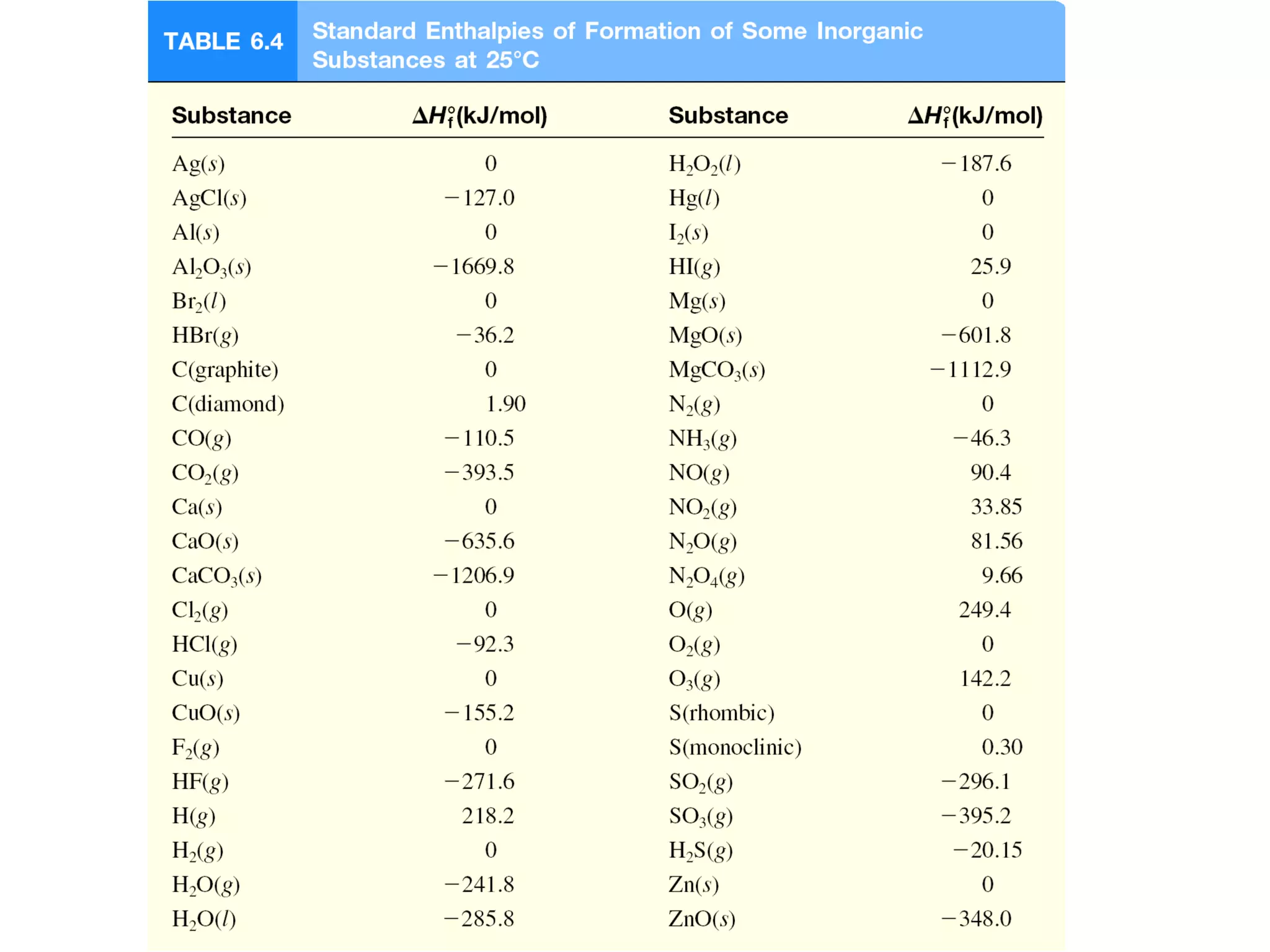
![The Direct Method for Determining ∆H 0
• Calculation of the enthalpy of formation of solid
calcium oxide.
CaO(s) + CO2(g) CaCO3(s) ∆Ho
rxn = -177.8 kJ/mol
∆Ho
rxn = Σn∆Ho
f(products) - Σn∆Ho
f(reactants)
-177.8 kJ/mol =1 mol(-1206.9) - [1 mol(x) + 1 mol(-393.5)]
∆Hf
o
forCaO(s)= -635.6 kJ](https://image.slidesharecdn.com/chapter6notes-150901140908-lva1-app6892/75/Chapter-6-notes-40-2048.jpg)
![45
Benzene (C6H6) burns in air to produce carbon dioxide and
liquid water. How much heat is released per mole of
benzene combusted? The standard enthalpy of formation
of benzene is 49.04 kJ/mol.
2C6H6 (l) + 15O2 (g) 12CO2 (g) + 6H2O (l)
∆H0
rxn n∆H0
(products)f= Σ m∆H0
(reactants)fΣ-
∆H0
rxn 6∆H0
(H2O)f12∆H0
(CO2)f= [ + ] - 2∆H0
(C6H6)f[ ]
∆H0
rxn = [ 12x–393.5 + 6x–187.6 ] – [ 2x49.04 ] = -5946 kJ
-5946 kJ
2 mol
= - 2973 kJ/mol C6H6](https://image.slidesharecdn.com/chapter6notes-150901140908-lva1-app6892/75/Chapter-6-notes-45-2048.jpg)