Embed presentation
Downloaded 80 times






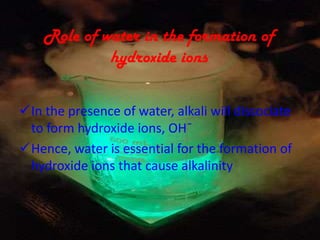
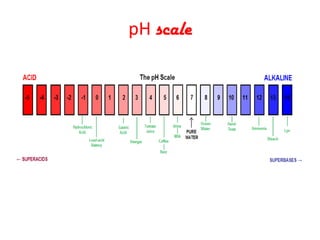








This document discusses acids and bases. It defines acids as substances that produce hydrogen ions in water and bases as substances that react with acids to form salts and water. It describes the chemical properties of acids and bases, including their tastes, pH levels, and ability to conduct electricity. Common acids like sulfuric acid and bases like sodium hydroxide are discussed along with their uses. The roles of water in producing hydrogen and hydroxide ions are explained. The pH scale is introduced as a measure of acidity and alkalinity. Strong and weak acids and bases are differentiated based on their ionization in water.















