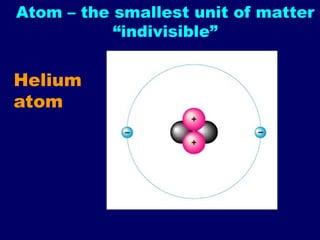
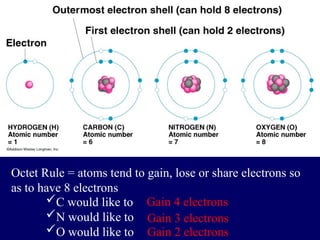
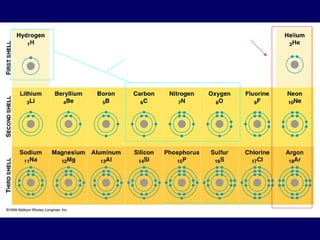
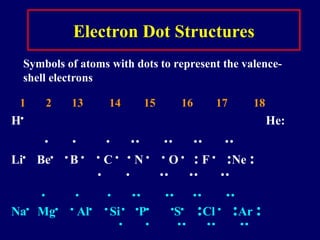
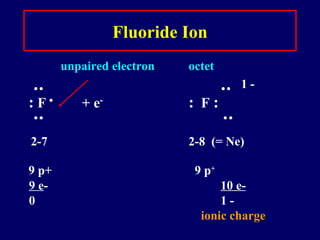
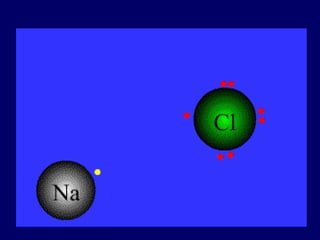
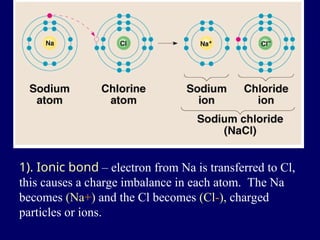
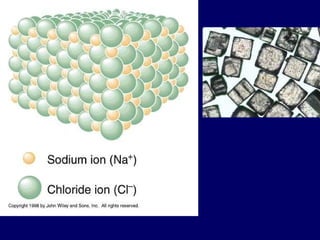
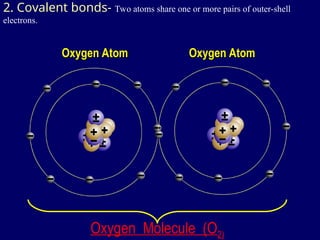
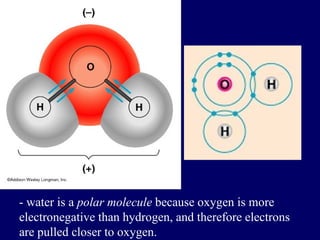
The document provides an overview of atomic structure, including the concept of atoms, electron arrangements in shells, and the formation of chemical bonds such as ionic, covalent, and metallic bonds. It explains the behavior of electrons in relation to the octet rule, the ionization of metals and nonmetals, and the various types of ions formed. Additionally, the document includes learning checks to assess understanding of valence electrons and ionic charges.