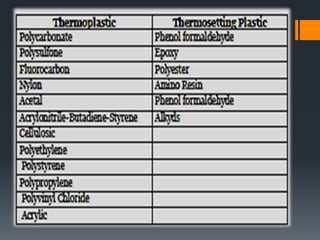
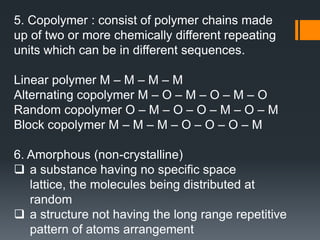
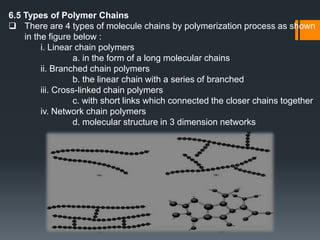
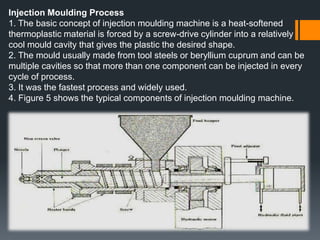
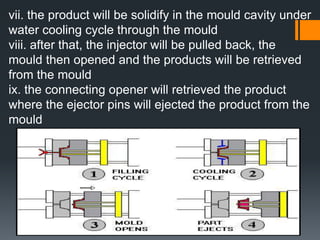
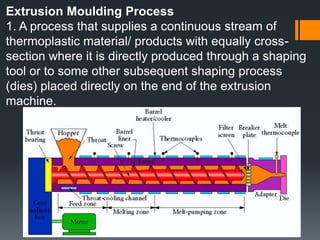
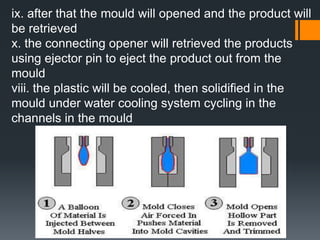
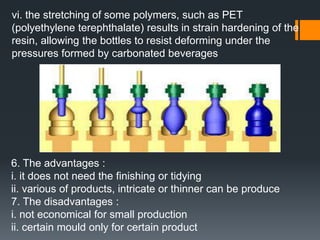
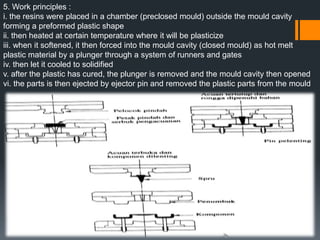
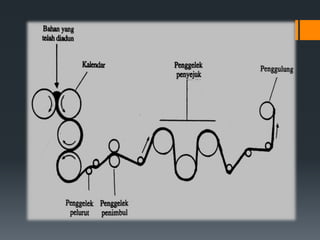
Plastic is a polymer material made from carbon and hydrogen that can be molded into shapes when heat and pressure are applied. Early plastics like celluloid and Bakelite were developed in the 1800s-1900s, but plastic use expanded in the 20th century. Plastics can be thermoplastics, which can be remelted and reshaped, or thermosets, which set permanently after initial molding. Common plastics are made through polymerization of monomers into chains or networks and have properties like strength, durability, and electrical insulation. Plastics are widely used for their processability, low cost, and ability to be formed into complex shapes.