The document discusses the Clausius theorem, which explains the second law of thermodynamics and the relationship between heat flow and entropy in reversible and irreversible processes. It outlines the inequality of Clausius, proofs regarding efficiencies of reversible and irreversible engines, and the concept of entropy change in open systems. Additionally, it defines reversible and irreversible processes, providing examples and factors that contribute to irreversibility.













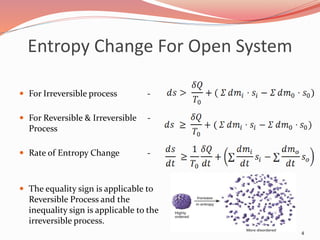
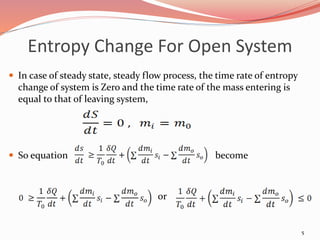
![Entropy Change For Open System
The general entropy balance
equation is :
[Rate of change of C.V.] = [Rate
of entropy transfer with heat] +
[Rate of entropy transport with
mass]
To = temperature of
surroundings
Si = specific entropy of the inlet
So = specific entropy of the
outlet
dmi = mass entering the system
dmo = mass leaving the system
2](https://image.slidesharecdn.com/150410119112-mech-161127064957/85/Entropy-14-320.jpg)