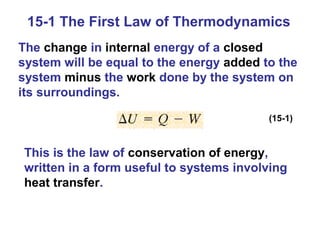
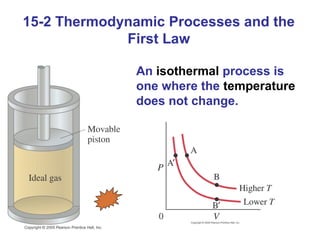
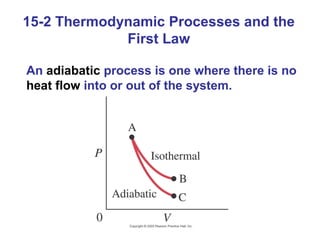
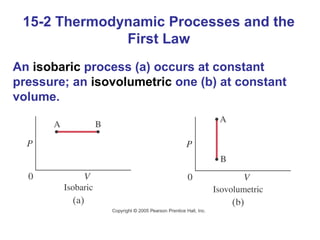
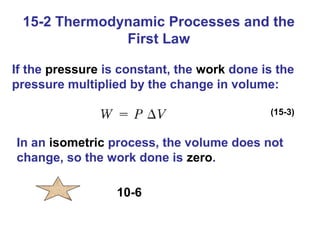
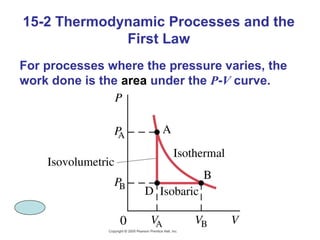
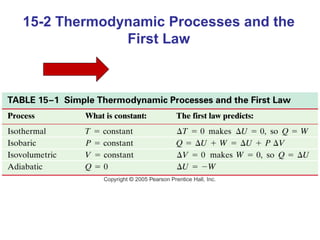
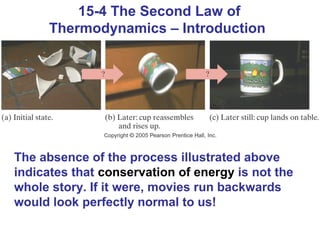
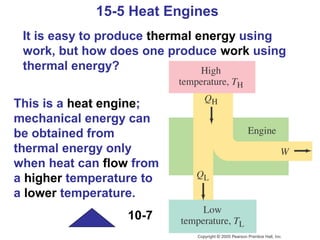
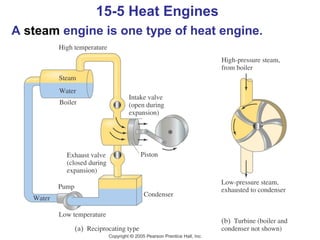
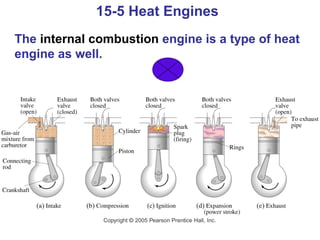
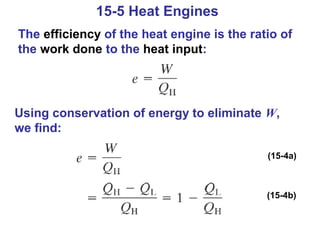
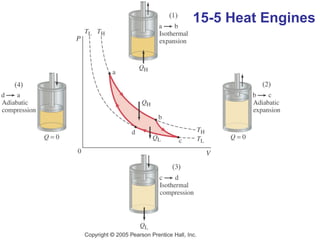
The document summarizes key concepts from Chapter 15 on thermodynamics. It introduces the first law of thermodynamics which states that the change in internal energy of a closed system equals the energy added minus work done. It then discusses different thermodynamic processes like isothermal, adiabatic, isobaric and isometric. The second law of thermodynamics is introduced which states that heat cannot spontaneously flow from a cold object to a hot object. Heat engines are then described which use a temperature difference to convert heat into work. The Carnot engine provides an ideal model to examine heat engine efficiency.