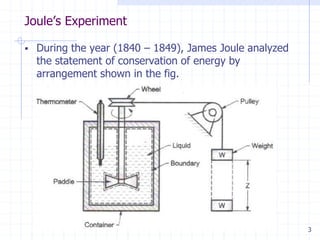
This document discusses the first law of thermodynamics and Joule's experiment that verified it. It contains the following key points:
1. Joule conducted an experiment in 1840-1849 where he used a paddle wheel apparatus to convert the potential energy of falling weights into heat energy in a liquid. He found that the work input was always proportional to the heat generated.
2. This established the first law of thermodynamics - that energy cannot be created or destroyed, only converted from one form to another.
3. The first law has consequences called corollaries, including that a perpetual motion machine of the first kind is impossible as it would produce work without an equivalent energy expenditure.