The document provides an overview of key concepts in thermodynamics from Chapter 10, including:
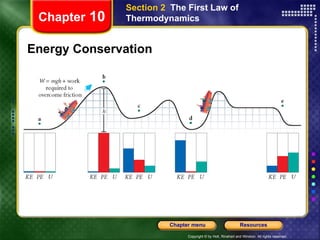
1) The first law of thermodynamics states that energy is conserved and relates heat, work, and changes in internal energy of a system.
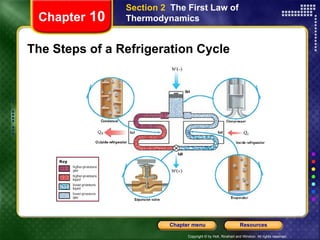
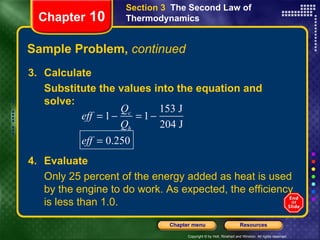
2) The second law states that no cyclic process can convert all heat into work. Heat engines and refrigerators operate cyclically between high and low temperatures.
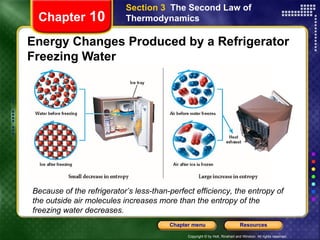
3) Entropy measures the disorder of a system. The second law can be expressed as the entropy of the universe always increasing in natural processes.