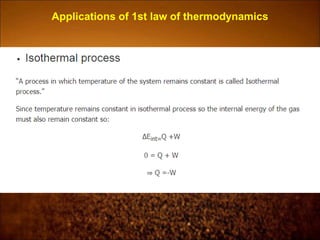
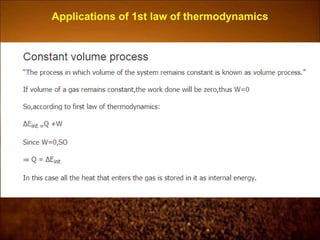
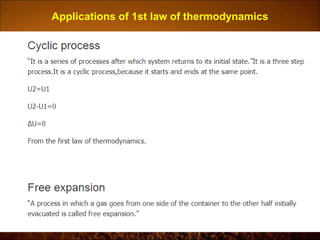
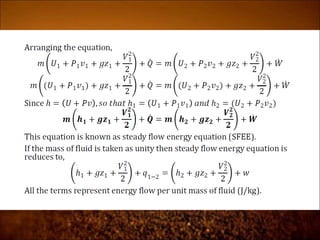
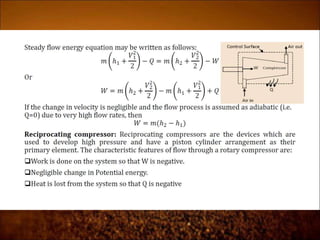
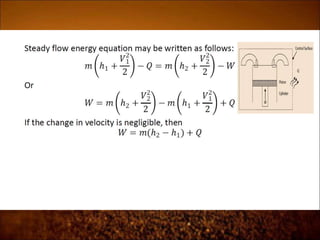
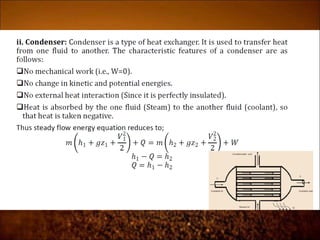
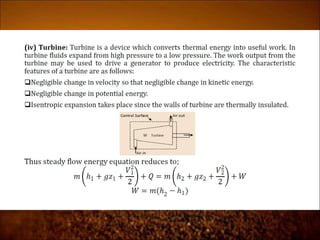
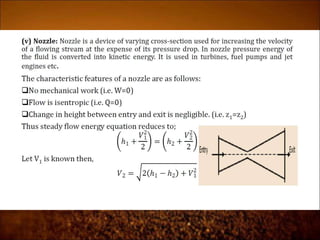
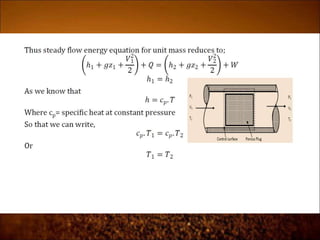
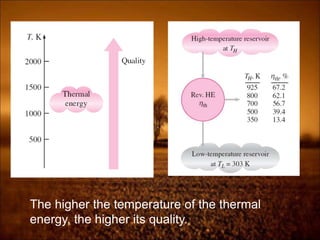
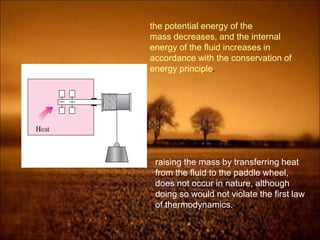
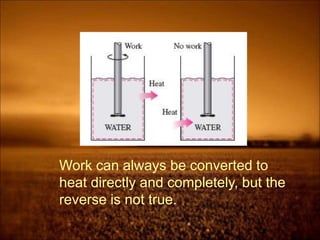
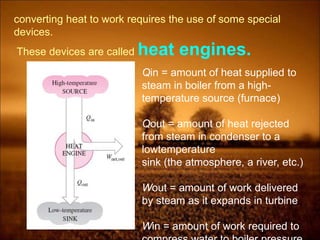
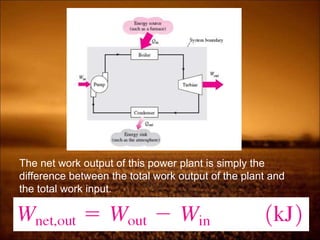
The document discusses the three laws of thermodynamics:
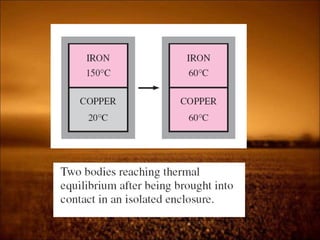
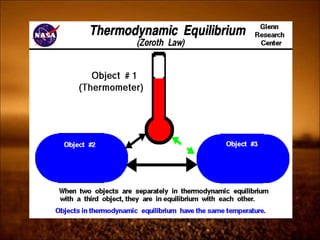
1. Zeroth law defines temperature and thermal equilibrium. It allows for temperature measurement.
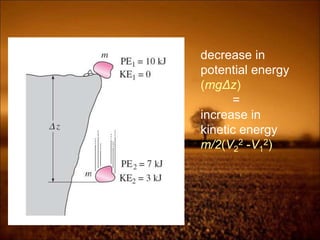
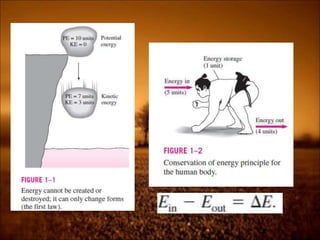
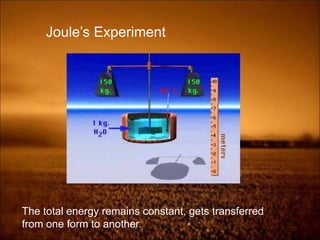
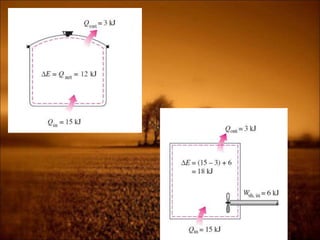
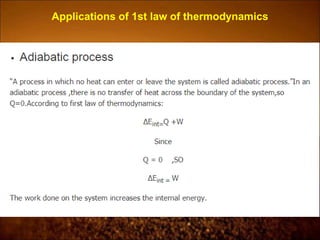
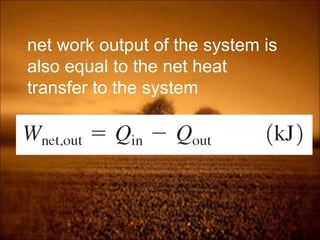
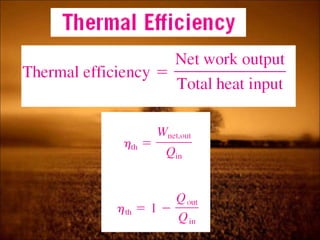
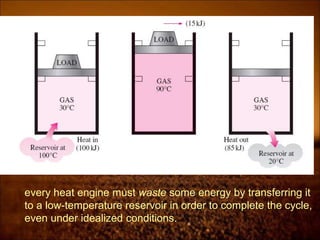
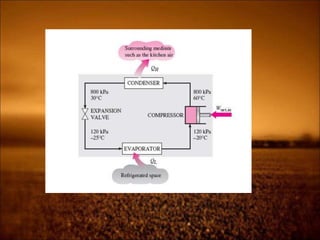
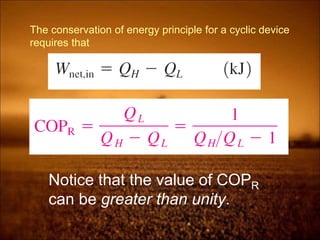
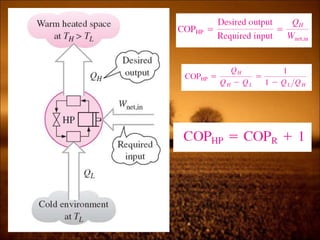
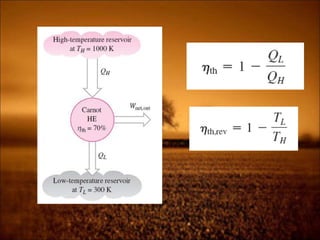
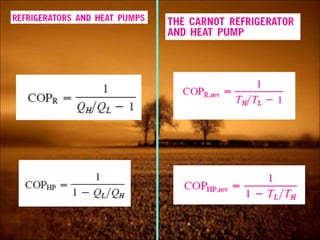
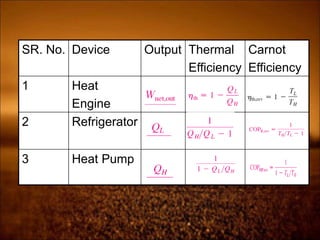
2. First law states that energy is conserved and can change forms but not be created or destroyed. It defines heat, work, and internal energy. Heat engines and refrigerators are discussed.
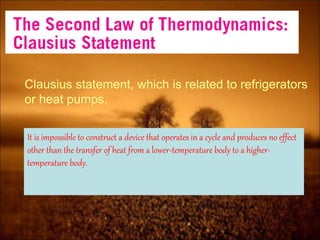
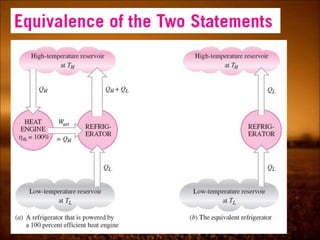
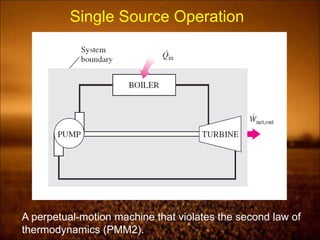
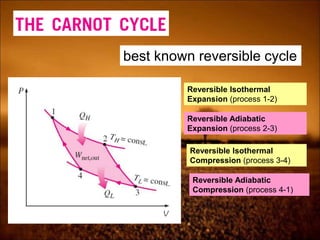
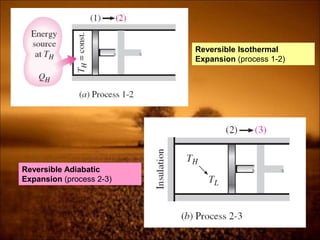
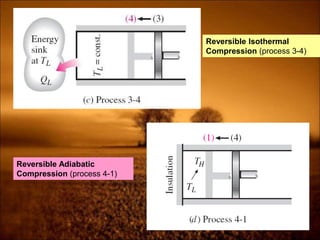
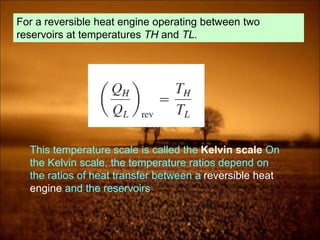
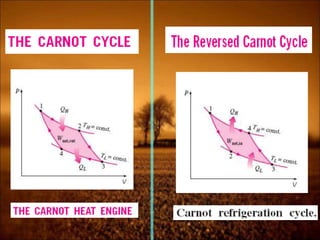
3. Second law introduces the concept of entropy and establishes limits on heat engine efficiency below 100% and prohibits converting heat to work without temperature difference. Carnot cycle and Carnot efficiency are introduced.
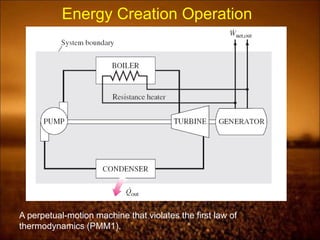
The laws establish fundamental thermodynamic principles and define key concepts like heat, work, efficiency. Processes must obey both the first and second laws or would create impossible