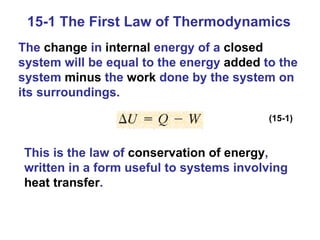
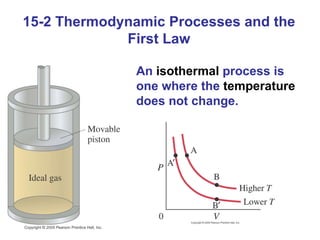
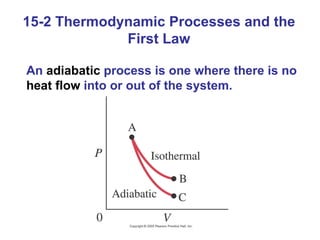
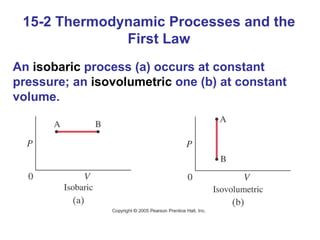
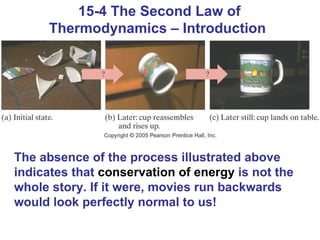
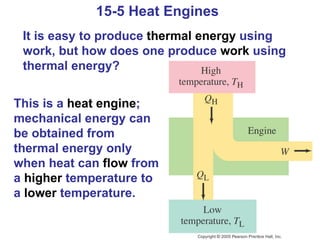
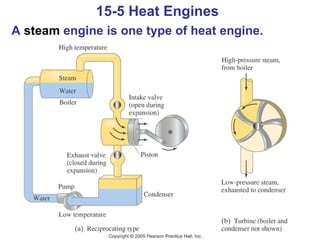
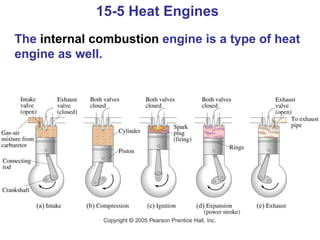
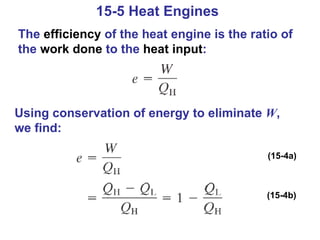
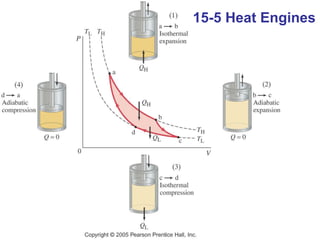
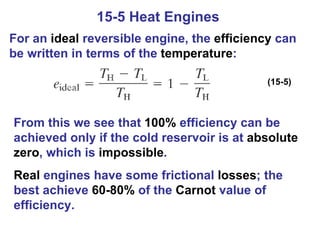
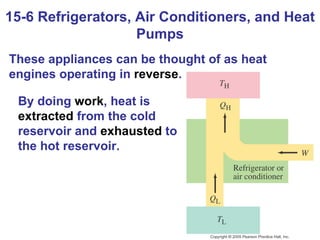
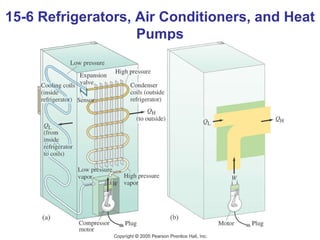
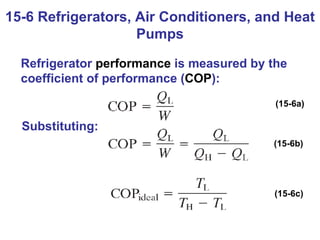
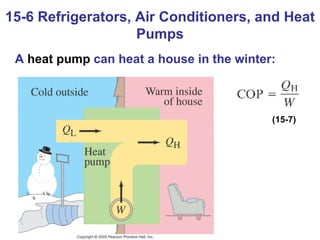
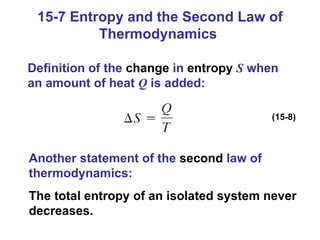
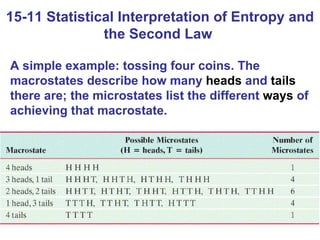
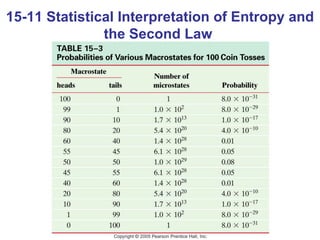
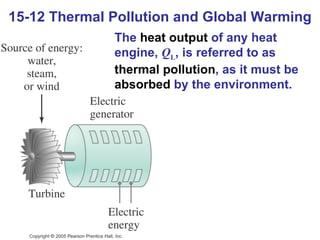
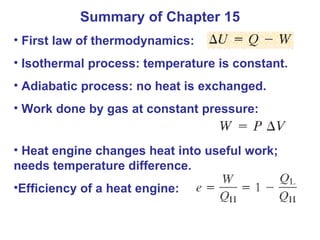
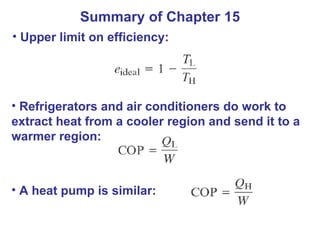
The document summarizes key concepts from Chapter 15 on thermodynamics. It discusses the first law of thermodynamics relating changes in internal energy to heat and work. The second law is introduced, stating that heat cannot spontaneously flow from cold to hot objects. Entropy is defined as a measure of disorder, and it is explained that entropy always increases for isolated systems, relating to the concept of the heat death of the universe.