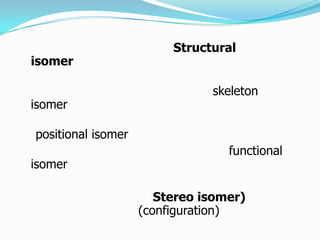
The document discusses several types of isomerism including structural isomers, stereoisomers, and optical isomers. It provides examples to illustrate structural isomers like positional and functional isomers. Stereoisomers include geometric isomers (cis-trans), optical isomers arising from chiral carbons, and diastereomers which are stereoisomers that are not mirror images. The document also discusses properties of meso compounds and uses Fischer projections to depict stereochemistry. Common organic reactions like substitution, addition, elimination, and oxidation are briefly overviewed with examples.